What Are The Two Starting Materials For A Robinson Annulation
Holbox
Mar 10, 2025 · 6 min read
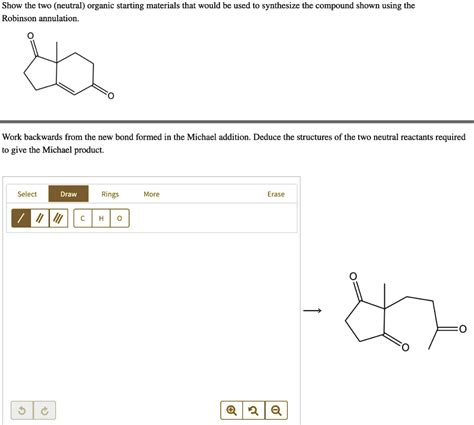
Table of Contents
What are the Two Starting Materials for a Robinson Annulation?
The Robinson annulation is a powerful and elegant one-pot reaction in organic chemistry, forming six-membered rings in a single synthetic step. Understanding its mechanism and the essential starting materials is crucial for anyone working with this versatile reaction. This article dives deep into the Robinson annulation, exploring the two crucial starting materials, their roles, and variations within the reaction. We will also touch upon the reaction mechanism and some important considerations for successful synthesis.
Understanding the Robinson Annulation: A Powerful Ring-Forming Reaction
The Robinson annulation, named after Robert Robinson, is a classic example of a domino reaction. It involves a Michael addition followed by an intramolecular aldol condensation to yield a six-membered ring system containing a carbonyl group and an unsaturated side chain. This reaction is exceptionally valuable in organic synthesis, particularly in the construction of complex polycyclic structures commonly found in natural products and pharmaceuticals. Its efficiency and simplicity make it a cornerstone technique in many organic chemistry laboratories.
The Two Essential Starting Materials: A Detailed Look
The Robinson annulation requires two key starting materials:
-
A α,β-unsaturated ketone (enone): This molecule acts as the Michael acceptor in the first step of the reaction. The α,β-unsaturation is crucial for the Michael addition to proceed efficiently. The carbonyl group is later involved in the aldol condensation. Common examples include methyl vinyl ketone (MVK), mesityl oxide, and other substituted enones. The choice of enone significantly impacts the final product's structure and properties.
-
A ketone containing at least one α-hydrogen: This is the Michael donor. The α-hydrogen is essential for the formation of the enolate ion, which initiates the Michael addition. The ketone's structure plays a crucial role in determining the regioselectivity and stereoselectivity of the reaction. Cyclohexanone, acetone, and other cyclic and acyclic ketones are commonly used. The presence of additional functional groups in the ketone can influence the course of the reaction and potentially lead to side products.
1. The α,β-Unsaturated Ketone: A Closer Examination
The α,β-unsaturated ketone is the cornerstone of the Robinson annulation's Michael acceptor role. The conjugated double bond system facilitates nucleophilic attack. Various substituents on this enone can alter the reaction's reactivity and selectivity. For instance:
-
Alkyl substitution: Alkyl groups at the α or β positions of the enone affect the steric hindrance, influencing the rate of the Michael addition. Bulky alkyl substituents can slow the reaction down.
-
Electron-withdrawing groups: These groups on the enone enhance its electrophilicity, making the Michael addition faster.
-
Electron-donating groups: Conversely, electron-donating groups decrease the enone's electrophilicity, thus slowing the reaction.
Choosing the appropriate α,β-unsaturated ketone is paramount for optimal yields and selectivity. This selection often depends on the desired final product's structure. Careful consideration of the steric and electronic effects of substituents is crucial in designing a successful Robinson annulation reaction.
2. The Ketone with α-Hydrogens: Unveiling Its Role
The ketone containing α-hydrogens serves as the Michael donor. The acidic α-hydrogen is essential for enolate formation under basic conditions. The enolate ion acts as the nucleophile, attacking the β-carbon of the α,β-unsaturated ketone. Several factors related to the ketone affect the outcome of the Robinson annulation:
-
Steric hindrance: Bulky substituents around the α-carbon can slow down enolate formation and hinder the Michael addition.
-
Electronic effects: Electron-withdrawing groups near the α-carbon can reduce the acidity of the α-hydrogen, affecting enolate formation. Electron-donating groups have the opposite effect.
-
Ring size: When cyclic ketones are used, the ring size can influence the reaction's stereochemistry and yield. Five and six-membered rings are commonly employed, often leading to specific stereochemical outcomes.
Understanding these aspects of the ketone's structure is critical for optimizing the reaction conditions and maximizing the yield of the desired product.
The Mechanism: A Step-by-Step Breakdown
The Robinson annulation unfolds through a two-step mechanism:
-
Michael Addition: The enolate ion formed from the ketone's α-hydrogen attacks the β-carbon of the α,β-unsaturated ketone. This step forms a new carbon-carbon bond, creating a β-keto ester intermediate. This intermediate is generally not isolated, as the next step proceeds rapidly.
-
Aldol Condensation: The intermediate then undergoes an intramolecular aldol condensation. The α-hydrogen of the β-keto ester reacts with the carbonyl group of the intermediate, leading to the formation of a new carbon-carbon bond and a six-membered ring. Dehydration then typically occurs, producing the conjugated enone system that characterizes the final Robinson annulation product.
Variations and Modifications of the Robinson Annulation
The Robinson annulation is not limited to a specific set of starting materials or conditions. Chemists have developed numerous modifications to enhance its scope and applicability:
-
Solvent effects: Different solvents can influence the reaction rate and selectivity. Polar aprotic solvents are often preferred.
-
Base selection: The choice of base significantly impacts the reaction's outcome. Commonly used bases include sodium hydroxide, potassium tert-butoxide, and sodium ethoxide. The base's strength and steric hindrance can influence the reaction's selectivity.
-
Temperature control: Optimizing the reaction temperature is essential. Too low a temperature can slow the reaction significantly, while excessive heat can lead to side reactions or decomposition of the reactants.
Applications of the Robinson Annulation: Beyond the Basics
The versatility of the Robinson annulation makes it an invaluable tool in numerous synthetic applications:
-
Natural product synthesis: The reaction has been widely used to synthesize various complex natural products, such as steroids and terpenoids. Its ability to build polycyclic structures efficiently makes it a cornerstone in many total synthesis strategies.
-
Pharmaceutical chemistry: The Robinson annulation plays a critical role in the synthesis of numerous pharmaceutical compounds. Its ability to create specific ring systems and functionalities makes it a valuable tool in drug discovery and development.
-
Material science: The reaction's ability to build complex molecular architectures also finds applications in the synthesis of novel materials with specific properties.
Conclusion: Mastering the Robinson Annulation
The Robinson annulation stands as a powerful testament to the elegance and efficiency of organic chemistry. Understanding the two starting materials, their roles, and the subtle factors affecting their reactivity is crucial for success in applying this reaction. With careful consideration of the starting materials, reaction conditions, and variations, the Robinson annulation can be employed to efficiently construct complex molecular architectures for a wide variety of synthetic endeavors. As demonstrated throughout this article, it remains a vital tool in the organic chemist's toolbox. The versatility and power of the Robinson annulation continue to make it a subject of ongoing research and development, expanding its potential applications in diverse fields. From natural product synthesis to pharmaceutical development and material science, this remarkable reaction continues to shape the landscape of modern organic chemistry.
Latest Posts
Latest Posts
-
Documenting The Gba Plus Process Can Assist You With
Mar 10, 2025
-
What Is A Homonym For Soar
Mar 10, 2025
-
A Cashier Is Finishing A Transaction With A Customer
Mar 10, 2025
-
Hey Mom I Finished That Book About Jennifer
Mar 10, 2025
-
Documents Are Marked With A Number And Then A Name
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about What Are The Two Starting Materials For A Robinson Annulation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
