Weak Acid And Weak Base Titration
Holbox
Mar 09, 2025 · 6 min read
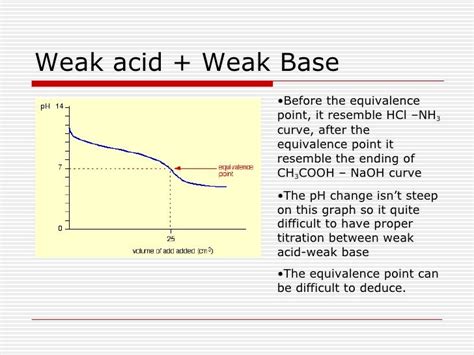
Table of Contents
Weak Acid and Weak Base Titration: A Comprehensive Guide
Titration is a fundamental analytical technique used extensively in chemistry to determine the concentration of an unknown solution (analyte) using a solution of known concentration (titrant). While strong acid-strong base titrations are relatively straightforward, weak acid-weak base titrations present unique challenges and complexities. This comprehensive guide delves into the intricacies of weak acid and weak base titrations, exploring the underlying principles, calculations, and practical considerations involved.
Understanding Weak Acids and Weak Bases
Before diving into the titration process, it's crucial to grasp the fundamental concepts of weak acids and weak bases. Unlike strong acids (e.g., HCl, HNO₃) and strong bases (e.g., NaOH, KOH), which completely dissociate in water, weak acids and bases only partially dissociate. This partial dissociation leads to an equilibrium between the undissociated acid/base and its ions.
Weak Acid Dissociation:
A weak acid, denoted as HA, partially dissociates in water according to the following equilibrium:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The equilibrium constant for this reaction is the acid dissociation constant, Ka, which is a measure of the acid's strength. A smaller Ka value indicates a weaker acid.
Weak Base Dissociation:
Similarly, a weak base, denoted as B, partially dissociates in water:
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
The equilibrium constant for this reaction is the base dissociation constant, Kb. A smaller Kb value indicates a weaker base.
The relationship between Ka and Kb for a conjugate acid-base pair is given by:
Ka * Kb = Kw
where Kw is the ion product constant of water (1.0 x 10⁻¹⁴ at 25°C).
The Titration Curve: A Visual Representation
The progress of a weak acid-weak base titration is best represented graphically by a titration curve. This curve plots the pH of the solution against the volume of titrant added. Unlike strong acid-strong base titrations which exhibit a sharp pH change at the equivalence point, weak acid-weak base titrations show a more gradual change.
Several key features characterize the titration curve:
-
Initial pH: The pH of the weak acid/base solution before any titrant is added. This pH is determined by the acid/base dissociation constant and the initial concentration.
-
Buffer Region: A region where the pH changes relatively slowly with the addition of titrant. This region is observed before the equivalence point and is due to the presence of a buffer solution containing both the weak acid/base and its conjugate.
-
Half-Equivalence Point: The point at which half of the weak acid/base has been neutralized. At this point, the pH = pKa for a weak acid titration or pH = pKb for a weak base titration. This is particularly useful for determining the pKa or pKb experimentally.
-
Equivalence Point: The point at which the moles of acid equal the moles of base. The pH at the equivalence point is not 7, unlike strong acid-strong base titrations. For a weak acid-strong base titration, the pH will be greater than 7, and for a weak base-strong acid titration, the pH will be less than 7.
-
Post-Equivalence Point: The region after the equivalence point where the pH changes more rapidly due to the excess of strong acid/base.
Calculations in Weak Acid-Weak Base Titration
Calculating the pH at different points in a weak acid-weak base titration requires a more nuanced approach compared to strong acid-strong base titrations. The following steps are typically involved:
1. Before the Equivalence Point:
The pH is determined by the equilibrium of the weak acid/base and its conjugate. The Henderson-Hasselbalch equation is frequently used:
For a weak acid titration: pH = pKa + log([A⁻]/[HA])
For a weak base titration: pOH = pKb + log([BH⁺]/[B])
Where [A⁻] and [HA] represent the concentrations of the conjugate base and weak acid, and [BH⁺] and [B] represent the concentrations of the conjugate acid and weak base, respectively.
2. At the Equivalence Point:
The pH at the equivalence point is determined by the hydrolysis of the conjugate base (weak acid titration) or conjugate acid (weak base titration). This requires considering the equilibrium of the conjugate with water and solving for the resulting hydroxide or hydronium ion concentration.
3. After the Equivalence Point:
The pH is determined by the excess of the strong acid or base added. The excess concentration is calculated and then used to determine the pH.
Practical Considerations and Limitations
Several practical considerations are crucial for accurate weak acid-weak base titrations:
-
Indicator Selection: Choosing the appropriate indicator is critical because the equivalence point pH is not 7. The indicator's pH range should encompass the pH at the equivalence point to ensure accurate determination.
-
Temperature Control: Temperature influences the dissociation constants (Ka and Kb) and thus affects the pH measurements. Maintaining a consistent temperature throughout the titration is important.
-
Solubility: The solubility of the weak acid or base in the solvent can impact the titration's accuracy. Ensuring complete dissolution is crucial.
-
Interferences: The presence of other substances in the solution might interfere with the titration. Careful sample preparation and consideration of potential interferences are essential.
Applications of Weak Acid-Weak Base Titration
Weak acid-weak base titrations find applications in various fields, including:
-
Pharmaceutical Analysis: Determining the purity and concentration of drugs that are weak acids or bases.
-
Environmental Monitoring: Analyzing water samples for the presence of weak acids or bases, like pollutants.
-
Food and Beverage Industry: Assessing the acidity or basicity of food and beverage products.
-
Agricultural Chemistry: Analyzing soil samples to determine the pH and nutrient levels.
Advanced Techniques and Considerations
Beyond the basic principles, more advanced techniques and concepts can enhance the accuracy and understanding of weak acid-weak base titrations:
-
Potentiometric Titration: Utilizing a pH meter for precise pH measurement instead of relying on visual indicators. This provides a more accurate determination of the equivalence point.
-
Gran Plot Analysis: A graphical method used to determine the equivalence point from potentiometric titration data, particularly useful for weak acid-weak base titrations where the equivalence point may not be sharply defined.
-
Non-Aqueous Titrations: For analytes that are not soluble in water, non-aqueous solvents are used. This expands the scope of weak acid-weak base titrations to a wider range of compounds.
-
Derivative Titration: This advanced technique utilizes the first or second derivative of the titration curve to pinpoint the equivalence point with greater accuracy.
Conclusion
Weak acid-weak base titrations are more intricate than strong acid-strong base titrations, demanding a deeper understanding of equilibrium principles and careful consideration of various factors. While presenting challenges, the successful execution of these titrations provides valuable insights into the properties and concentrations of weak acids and bases across diverse scientific disciplines. Mastering these techniques equips researchers and analysts with a powerful tool for quantitative analysis, unlocking a deeper understanding of chemical systems. The use of advanced techniques and careful attention to detail are key to achieving accurate and reliable results.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does Zn Have
Mar 10, 2025
-
How Do You Say Go To In Spanish
Mar 10, 2025
-
Eating Pork In The New Testament
Mar 10, 2025
-
Is The Nightingale A True Story
Mar 10, 2025
-
13 Out Of 16 As A Percentage
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Weak Acid And Weak Base Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
