Water Is A Polar Molecule Meaning It Carries Partial Charges
Holbox
Mar 22, 2025 · 6 min read
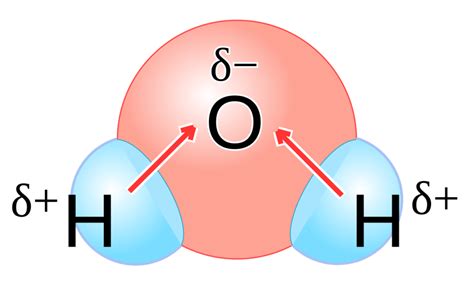
Table of Contents
- Water Is A Polar Molecule Meaning It Carries Partial Charges
- Table of Contents
- Water: A Polar Molecule and Its Significance
- Understanding Polarity: A Dipolar Moment
- The Bent Shape: Crucial for Polarity
- Consequences of Water's Polarity: A Cascade of Effects
- 1. Excellent Solvent: The Universal Solvent
- 2. High Surface Tension: Cohesion and Adhesion
- 3. High Specific Heat Capacity: Temperature Regulation
- 4. High Heat of Vaporization: Evaporative Cooling
- 5. Density Anomaly: Ice Floats
- Water's Polarity and Its Biological Significance
- Conclusion: The Profound Impact of a Polar Molecule
- Latest Posts
- Latest Posts
- Related Post
Water: A Polar Molecule and Its Significance
Water, the elixir of life, is far more than just a simple compound. Its unique properties, crucial for sustaining life as we know it, stem directly from its molecular structure: water is a polar molecule, meaning it carries partial charges. This seemingly simple fact has profound implications for its behavior and its role in biological and chemical processes. This article delves deep into the polarity of water, exploring its origins, consequences, and its impact on the world around us.
Understanding Polarity: A Dipolar Moment
To understand why water is a polar molecule, we must first grasp the concept of polarity. Polarity arises from differences in electronegativity between atoms within a molecule. Electronegativity is the ability of an atom to attract electrons in a chemical bond. In a water molecule (H₂O), the oxygen atom is significantly more electronegative than the hydrogen atoms.
This difference in electronegativity leads to an uneven distribution of electrons within the molecule. The oxygen atom attracts the shared electrons more strongly, creating a partial negative charge (δ-) near the oxygen atom. Conversely, the hydrogen atoms experience a partial positive charge (δ+). This uneven distribution of charge results in a dipole moment, a measure of the molecule's overall polarity. This dipole moment is what makes water a polar molecule. The molecule itself is not charged (it's electrically neutral overall), but it possesses distinct regions of positive and negative charge.
The Bent Shape: Crucial for Polarity
The shape of the water molecule is also crucial to its polarity. The water molecule is bent, not linear. If it were linear, the partial positive charges of the hydrogen atoms would cancel out the partial negative charge of the oxygen atom, resulting in a nonpolar molecule. However, the bent shape (approximately 104.5° bond angle) prevents this cancellation, ensuring that the molecule retains its dipole moment and its polar nature. This bent structure is a direct consequence of the presence of two lone pairs of electrons on the oxygen atom, which repel the bonding pairs and cause the bent geometry.
Consequences of Water's Polarity: A Cascade of Effects
The polarity of water is not simply an interesting fact; it is the foundation for many of water's unique properties, which are essential for life:
1. Excellent Solvent: The Universal Solvent
Water's polarity makes it an exceptional solvent, often called the "universal solvent." Polar molecules and ionic compounds readily dissolve in water because the partial charges in water molecules interact with the charges in these solutes. The positively charged ends of water molecules attract and surround negatively charged ions (anions), while the negatively charged ends surround positively charged ions (cations). This process, known as hydration, effectively surrounds and separates the ions, preventing them from re-associating and keeping them dissolved in solution.
This exceptional solvent property is crucial for numerous biological processes. It allows for the transport of nutrients and waste products in living organisms, facilitates chemical reactions within cells, and plays a vital role in maintaining the proper balance of electrolytes in the body.
2. High Surface Tension: Cohesion and Adhesion
The polarity of water leads to strong cohesion (attraction between water molecules) and adhesion (attraction between water molecules and other polar substances). These forces arise from hydrogen bonds, a special type of intermolecular force that occurs between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another.
The strong cohesive forces result in high surface tension, the tendency of water to minimize its surface area. This is why water forms droplets and allows insects to walk on water. The adhesive properties of water allow it to stick to surfaces, enabling capillary action, the movement of water against gravity in narrow tubes, which is crucial for water transport in plants.
3. High Specific Heat Capacity: Temperature Regulation
Water has a remarkably high specific heat capacity, meaning it can absorb a significant amount of heat energy without a large change in temperature. This is due to the extensive hydrogen bonding network in liquid water. A substantial amount of energy is needed to break these hydrogen bonds, which accounts for its ability to absorb heat energy without a significant temperature rise.
This property is essential for regulating temperature in both aquatic and terrestrial environments. Large bodies of water act as temperature buffers, preventing drastic temperature fluctuations that could harm aquatic life and terrestrial ecosystems. The human body also utilizes this property to maintain a stable internal temperature.
4. High Heat of Vaporization: Evaporative Cooling
Water also possesses a high heat of vaporization, meaning it requires a large amount of energy to change from a liquid to a gas (vaporization). This is again due to the strong hydrogen bonds that must be broken during evaporation. This high heat of vaporization allows for effective evaporative cooling. When water evaporates, it absorbs a significant amount of heat from its surroundings, leading to a cooling effect. This process is vital for temperature regulation in organisms, including humans (sweating) and plants (transpiration).
5. Density Anomaly: Ice Floats
Water exhibits a unique density anomaly: ice is less dense than liquid water. This unusual property is a direct consequence of the hydrogen bonding network. In ice, water molecules form a highly ordered crystalline structure with relatively large spaces between molecules. This open structure makes ice less dense than liquid water, causing ice to float on water.
This property has profound ecological consequences. Floating ice insulates the water below, preventing it from freezing solid and protecting aquatic life during winter. Without this anomaly, aquatic ecosystems would be severely affected.
Water's Polarity and Its Biological Significance
The polarity of water is not merely a physical property; it's a fundamental aspect that underpins life itself. Many crucial biological processes rely on water's polar nature:
-
Protein Folding: The three-dimensional structure of proteins, which dictates their function, is largely determined by interactions between water molecules and the polar and nonpolar regions of the protein.
-
Enzyme Activity: Many enzymes, which catalyze biochemical reactions, rely on water's polar nature to maintain their active sites and facilitate substrate binding.
-
Membrane Function: Cell membranes, composed of phospholipids, interact with water through their polar head groups, while the nonpolar tails are shielded from the aqueous environment.
-
DNA Structure: The double helix structure of DNA is stabilized by hydrogen bonds between the complementary bases, a type of interaction directly related to the polarity of water.
-
Nutrient Transport: Water's ability to dissolve polar molecules and ions is crucial for transporting nutrients throughout the body.
Conclusion: The Profound Impact of a Polar Molecule
The simple fact that water is a polar molecule has far-reaching consequences. Its polarity is the driving force behind many of its remarkable properties, which are essential for life on Earth. From its role as an excellent solvent to its influence on temperature regulation and its contribution to the stability of biological macromolecules, water's polarity underpins the delicate balance of life's processes. Understanding the polarity of water provides a crucial foundation for comprehending the chemistry of life and the intricate interactions that sustain our world. Further research continues to uncover new facets of water's behavior and its significance in various fields, from material science to climate modeling. The seemingly simple water molecule is a testament to the profound impact of fundamental chemical properties on the complexity of life and the environment.
Latest Posts
Latest Posts
-
Toxic Chemicals That Are Shipped To A Food Establishment Must
Mar 24, 2025
-
Nicholas Jones Is A Chiropractor In New York Npi Number
Mar 24, 2025
-
The Roosevelt Corollary To The Monroe Doctrine Asserted That
Mar 24, 2025
-
Which Of The Following Best Describes Angina Pectoris
Mar 24, 2025
-
Only A Person Could Believe Her Tale
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Water Is A Polar Molecule Meaning It Carries Partial Charges . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
