Use The Chart To Determine The Half-life Of Carbon-14.
Holbox
Mar 13, 2025 · 6 min read
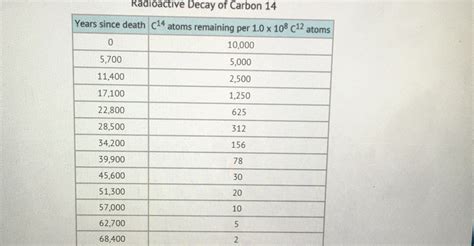
Table of Contents
- Use The Chart To Determine The Half-life Of Carbon-14.
- Table of Contents
- Determining the Half-Life of Carbon-14: A Comprehensive Guide
- Understanding Radioactive Decay and Half-Life
- Using a Decay Chart to Determine the Half-Life of Carbon-14
- Interpreting Decay Charts: Common Challenges and Considerations
- Applications of Carbon-14 Half-Life: Radiocarbon Dating
- Beyond Radiocarbon Dating: Other Applications of Carbon-14
- Conclusion: The Significance of Precise Half-Life Determination
- Latest Posts
- Related Post
Determining the Half-Life of Carbon-14: A Comprehensive Guide
Carbon-14, a radioactive isotope of carbon, plays a crucial role in various scientific fields, most notably in radiocarbon dating. Understanding its half-life is fundamental to accurately determining the age of ancient artifacts and fossils. This article delves into the process of determining the half-life of Carbon-14 using a decay chart, exploring the underlying principles of radioactive decay and the practical applications of this knowledge.
Understanding Radioactive Decay and Half-Life
Before we delve into using a chart to determine the half-life, let's solidify our understanding of the core concepts. Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation. This radiation can take several forms, including alpha particles, beta particles, and gamma rays. Carbon-14 undergoes beta decay, where a neutron transforms into a proton, emitting an electron (beta particle) and an antineutrino.
The half-life of a radioactive isotope is the time it takes for half of the atoms in a given sample to decay. This is a constant value for a specific isotope, regardless of the initial amount of the substance. It's crucial to understand that half-life isn't the time it takes for all the atoms to decay; rather, it's the time it takes for the amount to be reduced by half. After one half-life, half remains; after two half-lives, one-quarter remains; after three half-lives, one-eighth remains, and so on. This follows an exponential decay pattern.
Using a Decay Chart to Determine the Half-Life of Carbon-14
A decay chart, also known as a decay curve or a radioactive decay graph, visually represents the decrease in the number of radioactive atoms over time. The chart typically plots the number of radioactive atoms (or the activity, which is proportional to the number of atoms) on the y-axis and time on the x-axis. The curve resulting from plotting this data is exponential.
To determine the half-life from a decay chart, follow these steps:
-
Identify the initial amount: Find the starting point on the y-axis, representing the initial number of Carbon-14 atoms at time zero.
-
Find half the initial amount: Divide the initial amount by two. This represents the amount of Carbon-14 remaining after one half-life.
-
Locate half the initial amount on the y-axis: Find the point on the y-axis corresponding to half the initial amount.
-
Draw a horizontal line: Draw a horizontal line from this point across the graph until it intersects the decay curve.
-
Draw a vertical line: Draw a vertical line downwards from the intersection point to the x-axis.
-
Read the half-life: The point where the vertical line intersects the x-axis represents the time it took for half of the initial Carbon-14 atoms to decay, thus indicating the half-life.
Example:
Let's assume a hypothetical decay chart shows that we start with 1000 Carbon-14 atoms. After carefully examining the chart, we find that the number of atoms reduces to 500 after approximately 5730 years. Therefore, based on this chart, the half-life of Carbon-14 is approximately 5730 years. This is the accepted value used in radiocarbon dating.
Interpreting Decay Charts: Common Challenges and Considerations
While seemingly straightforward, interpreting decay charts can present challenges. Here are some crucial considerations:
-
Accuracy of the Chart: The accuracy of the determined half-life is directly dependent on the accuracy of the data used to construct the chart. Errors in measurement or data collection can lead to inaccuracies in the determined half-life.
-
Scale and Units: Pay close attention to the scales used on both axes. The units of time (years, days, etc.) and the units for the number of atoms or activity (Becquerels, Curies, etc.) need to be correctly identified to avoid misinterpretations.
-
Data Points: The more data points plotted on the chart, the more accurate the determination of the half-life becomes. A limited number of data points can lead to greater uncertainty.
-
Background Radiation: When dealing with real-world measurements, background radiation can interfere with the readings. This necessitates corrections and careful consideration of potential sources of error.
-
Statistical Fluctuations: At the atomic level, radioactive decay is a probabilistic process. Therefore, slight fluctuations in the measured decay rate are expected, especially with smaller sample sizes.
Applications of Carbon-14 Half-Life: Radiocarbon Dating
The accurate determination of Carbon-14's half-life is paramount to the technique of radiocarbon dating, a cornerstone of archaeology, anthropology, and geology. This method allows scientists to determine the age of organic materials up to approximately 50,000 years old.
The Principle of Radiocarbon Dating:
Living organisms constantly exchange carbon with their environment, maintaining a constant ratio of Carbon-14 to Carbon-12. However, once an organism dies, this exchange ceases. The Carbon-14 within the organism begins to decay at a known rate (determined by its half-life), while the Carbon-12 remains stable. By measuring the ratio of Carbon-14 to Carbon-12 in a sample, scientists can estimate the time elapsed since the organism died.
Limitations of Radiocarbon Dating:
While incredibly useful, radiocarbon dating has limitations:
-
Contamination: Contamination of the sample with younger or older carbon can significantly affect the results. Careful sample preparation and cleaning are crucial.
-
Reservoir Effects: Variations in the atmospheric concentration of Carbon-14 over time can influence dating accuracy. Scientists use calibration curves to correct for these variations.
-
Age Range: The technique is most reliable for samples within a specific age range (roughly 50,000 years). Beyond this, the remaining Carbon-14 becomes too small to measure accurately.
Beyond Radiocarbon Dating: Other Applications of Carbon-14
While radiocarbon dating is the most prominent application, Carbon-14's half-life and radioactive properties find use in other areas:
-
Medical Research: Carbon-14 is used as a tracer in various medical studies, allowing researchers to track the movement and metabolism of compounds within the body.
-
Environmental Science: Carbon-14 is used to study environmental processes such as groundwater flow and carbon cycling in ecosystems.
-
Industrial Applications: Carbon-14 is employed in certain industrial processes, including the measurement of wear and tear in mechanical components.
Conclusion: The Significance of Precise Half-Life Determination
The accurate determination of the half-life of Carbon-14 is not merely an academic exercise; it forms the very foundation of crucial scientific techniques like radiocarbon dating. Understanding the principles of radioactive decay, correctly interpreting decay charts, and acknowledging potential sources of error are all essential for ensuring the reliability and accuracy of results obtained using Carbon-14. This knowledge allows scientists to unravel the mysteries of the past, understand complex biological processes, and monitor environmental changes with greater precision. The ongoing refinement of techniques and understanding of Carbon-14's behavior continues to expand our knowledge across diverse scientific disciplines. The ability to accurately determine and utilize the half-life of this isotope is a testament to the power of scientific inquiry and its profound impact on our world.
Latest Posts
Related Post
Thank you for visiting our website which covers about Use The Chart To Determine The Half-life Of Carbon-14. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.