University Of Colorado Phet Concentration Exercise
Holbox
Mar 27, 2025 · 6 min read
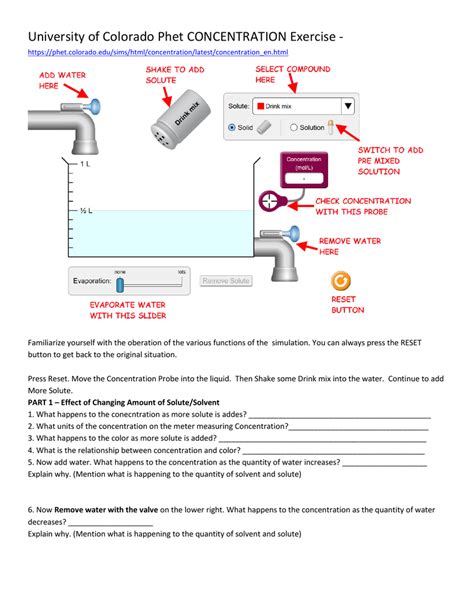
Table of Contents
- University Of Colorado Phet Concentration Exercise
- Table of Contents
- University of Colorado PhET Concentration Exercise: A Deep Dive into Interactive Learning
- Understanding the PhET Concentration Simulation
- Key Features and Functionality
- Educational Applications and Pedagogical Implications
- Enhancing Conceptual Understanding
- Catering to Diverse Learning Styles
- Fostering Critical Thinking and Problem Solving
- Integrating with Curriculum
- Addressing Common Misconceptions
- Advanced Applications and Extensions
- Exploring Saturation and Solubility
- Investigating the Effects of Temperature
- Connecting to Real-World Applications
- Assessment and Evaluation
- Pre- and Post-Simulation Quizzes
- Observation and Discussion
- Open-Ended Questions and Problem Solving
- Conclusion: PhET Concentration – A Catalyst for Effective Learning
- Latest Posts
- Latest Posts
- Related Post
University of Colorado PhET Concentration Exercise: A Deep Dive into Interactive Learning
The University of Colorado Boulder's PhET Interactive Simulations are renowned for their ability to transform abstract scientific concepts into engaging, interactive experiences. Among their vast collection, the "Concentration" simulation stands out as a particularly effective tool for exploring the principles of concentration, solubility, and solution chemistry. This article delves deep into the PhET Concentration exercise, exploring its features, educational applications, and pedagogical implications. We'll unpack how this simulation can enhance learning, catering to diverse learning styles and fostering a deeper understanding of complex chemical concepts.
Understanding the PhET Concentration Simulation
The PhET Concentration simulation provides a virtual laboratory environment where users can experiment with various solutions and observe the effects of changing solute and solvent amounts. Its intuitive interface allows users to manipulate variables, visualize changes in concentration, and directly connect experimental observations to theoretical understanding. This hands-on, experiential approach is crucial for mastering concepts often found abstract and challenging in traditional textbook learning.
Key Features and Functionality
The simulation offers several key features that make it a powerful learning tool:
-
Adjustable Parameters: Users can adjust the amount of solute (the substance being dissolved) and solvent (the substance doing the dissolving) independently, allowing for precise control over solution composition. This direct manipulation fosters a strong understanding of concentration’s dependence on these variables.
-
Visual Representation: The simulation visually represents the solution at a molecular level, showing the solute particles dissolving in the solvent. This visual representation aids in conceptual understanding by connecting macroscopic observations with microscopic behavior. Students can directly observe how changing the amounts of solute and solvent affects the particle density and subsequently, the concentration.
-
Multiple Concentration Units: The simulation supports multiple concentration units, including molarity, molality, and percent by mass. This feature is particularly valuable as it allows students to transition smoothly between different concentration expressions, emphasizing their interrelationship and providing a solid foundation for future coursework.
-
Interactive Experiments: The simulation encourages experimentation through its intuitive interface. Students can freely manipulate variables, observe outcomes, and deduce relationships between concentration and other factors. This self-directed exploration fosters critical thinking and problem-solving skills.
-
Data Recording and Analysis: Though not explicitly built-in as a data table, the simulation allows for data collection through observation and mental note-taking. Students can easily record the amount of solute and solvent added, the resulting concentration displayed, and their observations regarding the solution’s appearance. This aspect allows students to effectively practice the scientific method, encouraging them to develop hypotheses, conduct experiments, and analyze data to arrive at conclusions.
Educational Applications and Pedagogical Implications
The PhET Concentration simulation is not just a fun interactive tool; it's a powerful educational resource with broad applications across various educational levels.
Enhancing Conceptual Understanding
The simulation effectively addresses many challenges associated with teaching concentration. The abstract nature of molarity, molality, and percent composition often proves difficult for students to grasp. By visually representing the solution at a molecular level and allowing students to manipulate the variables directly, the simulation bridges the gap between abstract concepts and concrete visualizations, leading to a stronger conceptual understanding.
Catering to Diverse Learning Styles
The simulation's interactive nature caters to diverse learning styles. Visual learners benefit from the dynamic molecular visualization, while kinesthetic learners appreciate the hands-on manipulation of variables. Auditory learners can discuss their observations and interpretations with peers or instructors, enhancing their learning experience. This multifaceted approach ensures that the simulation can effectively engage a wider range of students.
Fostering Critical Thinking and Problem Solving
The simulation's open-ended nature encourages critical thinking and problem-solving. Students are not merely passively absorbing information; they are actively engaging with the material, formulating hypotheses, conducting experiments, and analyzing results. This active learning approach fosters a deeper and more meaningful understanding of the concepts.
Integrating with Curriculum
The PhET Concentration simulation can be seamlessly integrated into various curriculum levels. It is an ideal supplement to traditional textbook learning, laboratory experiments, and classroom discussions. Its versatility allows instructors to tailor its usage to specific learning objectives and student needs. It can be used as an introductory activity to introduce the concept of concentration, as a reinforcement activity to consolidate learning, or as an assessment tool to gauge student understanding.
Addressing Common Misconceptions
Many students harbor misconceptions about solution chemistry, such as confusing concentration with the amount of solute or solvent. The simulation helps address these misconceptions by allowing students to experiment and directly observe the relationships between these variables. Students can manipulate the amounts of solute and solvent while observing the calculated concentration, helping them form a clearer understanding of how these quantities relate.
Advanced Applications and Extensions
The basic features of the simulation provide a strong foundation, but its applications can be extended to explore more advanced topics.
Exploring Saturation and Solubility
By gradually increasing the amount of solute, students can observe the concept of saturation and solubility. They can experimentally determine the solubility limit of a given solute in a specific solvent at a particular temperature, solidifying their understanding of these crucial concepts.
Investigating the Effects of Temperature
While the basic simulation doesn't include temperature as a variable, instructors can use the simulation as a springboard to discuss the impact of temperature on solubility. This can spark discussions and lead to further research and investigations.
Connecting to Real-World Applications
The simulation provides an excellent opportunity to connect abstract concepts to real-world applications. Discussions about the relevance of concentration in various fields such as medicine, environmental science, and food science can enhance students' engagement and understanding. For example, discussing the concentration of medication in the bloodstream or the concentration of pollutants in water bodies can bring a tangible relevance to the simulated experiments.
Assessment and Evaluation
Effectively assessing student learning with the PhET Concentration simulation necessitates a multi-faceted approach.
Pre- and Post-Simulation Quizzes
Simple quizzes administered before and after using the simulation can effectively measure the changes in student understanding. These quizzes can focus on key concepts like molarity, molality, percent composition, and solubility.
Observation and Discussion
Observing students while they interact with the simulation can provide valuable insights into their understanding and problem-solving abilities. Engaging students in discussions about their observations and interpretations further solidifies their understanding and allows for clarification of misconceptions.
Open-Ended Questions and Problem Solving
Asking students open-ended questions that require them to apply their knowledge of concentration to solve problems is a crucial step. For example, posing problems that involve calculating the concentration of a solution given specific amounts of solute and solvent, or determining the amount of solute needed to achieve a specific concentration, can assess their comprehensive understanding.
Conclusion: PhET Concentration – A Catalyst for Effective Learning
The University of Colorado's PhET Concentration simulation proves to be a powerful tool for teaching and learning solution chemistry. Its interactive nature, visual representations, and versatile features cater to diverse learning styles and foster a deeper understanding of complex concepts. By integrating this simulation into the curriculum, educators can create a more engaging, effective, and stimulating learning environment, ultimately enhancing students' understanding of concentration and its importance in various scientific disciplines. The simulation’s accessibility and ease of use further contribute to its value as a crucial resource for science education, offering a dynamic and interactive alternative to traditional methods. Its flexibility in application, from introductory concepts to advanced problem-solving, ensures its continued relevance in modern science education, providing a valuable contribution to effective learning and improved student outcomes.
Latest Posts
Latest Posts
-
Identify The Expected Major Product Of The Following Reaction
Mar 31, 2025
-
Object A Is Released From Rest At Height H
Mar 31, 2025
-
Which One Of The Following Is A Source Of Cash
Mar 31, 2025
-
The Contribution Margin Equals Sales Minus All
Mar 31, 2025
-
A Salad Bar Attendant Had To Replace
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about University Of Colorado Phet Concentration Exercise . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
