Thiols Have Structures Similar To Alcohols Except That They Contain
Holbox
Mar 14, 2025 · 6 min read
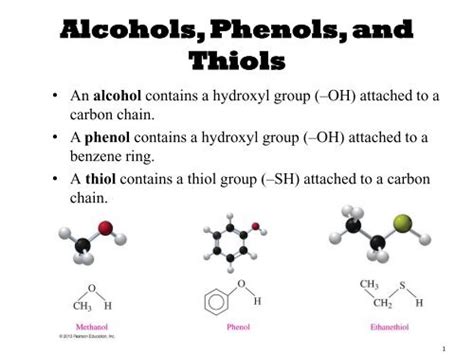
Table of Contents
- Thiols Have Structures Similar To Alcohols Except That They Contain
- Table of Contents
- Thiols: The Sulfur-Containing Cousins of Alcohols
- Structural Similarities and Differences Between Thiols and Alcohols
- The Sulfhydryl Group: The Heart of Thiol Chemistry
- Structural Variations in Thiols
- Physical Properties of Thiols: A Comparison with Alcohols
- Boiling Points: A Tale of Two Heteroatoms
- Solubility: The Impact of Polarity
- Odor: The Distinctive Smell of Thiols
- Chemical Reactivity of Thiols: Nucleophilic Prowess
- Thiols as Nucleophiles: A Range of Reactions
- Formation of Disulfides: A Crucial Reaction
- Synthesis of Thiols: Diverse Approaches
- From Alkyl Halides: Nucleophilic Substitution
- From Alcohols: Conversion via Thionyl Chloride
- From Alkenes: Addition of Hydrogen Sulfide
- Applications of Thiols: A Diverse Landscape
- Biochemistry: The Role in Proteins and Enzymes
- Industry: Applications in Diverse Sectors
- Medicine: Therapeutic Potential of Thiols
- Toxicity and Safety Considerations
- Conclusion: The Versatility of Thiols
- Latest Posts
- Related Post
Thiols: The Sulfur-Containing Cousins of Alcohols
Thiols, also known as mercaptans, are organic compounds that share a structural resemblance to alcohols but with a crucial difference: instead of an oxygen atom, they contain a sulfur atom bonded to a carbon atom. This seemingly small substitution leads to significant differences in their properties, reactivity, and applications. This article delves deep into the world of thiols, exploring their structure, properties, synthesis, reactivity, and diverse applications across various fields.
Structural Similarities and Differences Between Thiols and Alcohols
Both thiols and alcohols are characterized by the presence of a functional group bonded to a carbon atom. In alcohols, this functional group is a hydroxyl group (-OH), while in thiols, it's a sulfhydryl group (-SH). This seemingly minor difference in the heteroatom dramatically alters their chemical behavior.
The Sulfhydryl Group: The Heart of Thiol Chemistry
The sulfhydryl group (-SH) is the defining feature of thiols. The sulfur atom possesses two lone pairs of electrons and a larger atomic radius than oxygen. This contributes to the unique reactivity of thiols compared to alcohols. The larger size and greater polarizability of sulfur lead to weaker hydrogen bonding compared to alcohols. This impacts the physical properties like boiling points and solubility.
Structural Variations in Thiols
Thiols can exist as primary, secondary, or tertiary, depending on the number of carbon atoms attached to the carbon atom bearing the sulfhydryl group. Just like alcohols, the structure influences their reactivity and properties.
- Primary Thiols: The carbon atom bonded to the -SH group is attached to only one other carbon atom (R-CH2-SH).
- Secondary Thiols: The carbon atom bonded to the -SH group is attached to two other carbon atoms (R1R2CH-SH).
- Tertiary Thiols: The carbon atom bonded to the -SH group is attached to three other carbon atoms (R1R2R3C-SH).
Physical Properties of Thiols: A Comparison with Alcohols
While structurally similar to alcohols, thiols exhibit noticeably different physical properties. These differences stem primarily from the lower electronegativity of sulfur compared to oxygen, leading to weaker intermolecular forces.
Boiling Points: A Tale of Two Heteroatoms
Alcohols exhibit significantly higher boiling points than thiols of comparable molecular weight. This is due to the stronger hydrogen bonding in alcohols. The weaker hydrogen bonding in thiols results in lower boiling points.
Solubility: The Impact of Polarity
The solubility of thiols in water is generally lower than that of alcohols. While the -SH group is polar, the weaker polarity compared to the -OH group reduces its ability to interact favorably with water molecules. Larger thiols exhibit even lower solubility due to the increasing dominance of the hydrophobic alkyl chain.
Odor: The Distinctive Smell of Thiols
Many thiols are characterized by their strong, unpleasant odor, often described as "rotten eggs" or "skunky." This is attributed to the ease with which thiols can be detected by the human olfactory system, even at very low concentrations. This characteristic odor is crucial in many applications, including leak detection in gas pipelines.
Chemical Reactivity of Thiols: Nucleophilic Prowess
Thiols display a rich array of chemical reactions, primarily due to the nucleophilic nature of the sulfur atom in the sulfhydryl group. The lone pairs of electrons on the sulfur are readily available to participate in nucleophilic attack on electrophilic centers.
Thiols as Nucleophiles: A Range of Reactions
The nucleophilicity of thiols makes them versatile reagents in various organic reactions. Here are some key examples:
- Alkylation: Thiols readily undergo alkylation reactions, where the sulfhydryl group replaces a leaving group in an alkyl halide.
- Acylation: Thiols can be acylated to form thioesters, analogous to the formation of esters from alcohols.
- Oxidation: Thiols can be oxidized to form disulfides (-S-S-), a crucial process in protein structure and function.
- Addition to Carbonyl Compounds: Thiols can add to carbonyl compounds to form hemithioacetals and thioacetals.
Formation of Disulfides: A Crucial Reaction
The oxidation of thiols to disulfides is particularly important in biochemistry. This reaction is reversible and plays a vital role in the formation of disulfide bridges in proteins, contributing to their three-dimensional structure and stability.
Synthesis of Thiols: Diverse Approaches
Several methods exist for the synthesis of thiols, each offering unique advantages and limitations.
From Alkyl Halides: Nucleophilic Substitution
One common approach involves the nucleophilic substitution of an alkyl halide with a sulfur nucleophile, such as a thiolate ion (RS⁻). This reaction is often carried out in the presence of a base to generate the thiolate ion.
From Alcohols: Conversion via Thionyl Chloride
Alcohols can be converted to thiols through a multi-step process involving reaction with thionyl chloride (SOCl2) followed by reaction with a suitable sulfur nucleophile.
From Alkenes: Addition of Hydrogen Sulfide
Hydrogen sulfide (H2S) can add across the double bond of alkenes to yield thiols. This reaction requires specific conditions and catalysts to achieve high yields.
Applications of Thiols: A Diverse Landscape
Thiols find widespread applications in various fields, leveraging their unique chemical properties and reactivity.
Biochemistry: The Role in Proteins and Enzymes
Thiols play essential roles in numerous biological processes. Cysteine, an amino acid containing a thiol group, is crucial in protein structure and function, contributing to disulfide bond formation and enzymatic activity. Glutathione, a tripeptide containing a thiol group, acts as a potent antioxidant and detoxifying agent.
Industry: Applications in Diverse Sectors
Thiol applications extend beyond biochemistry. They are employed in various industrial processes:
- Petroleum Refining: Thiols are used as odorants in natural gas, providing an easily detectable warning of leaks.
- Polymer Chemistry: Thiols are used as chain-transfer agents in the polymerization of various monomers.
- Pharmaceuticals: Thiols are found in several drugs and are utilized in drug design and development.
- Agriculture: Certain thiols are used as pesticides and fungicides.
Medicine: Therapeutic Potential of Thiols
Thiols exhibit significant therapeutic potential. They are explored as potential treatments for various diseases, including cancer and neurodegenerative disorders. Their antioxidant and anti-inflammatory properties make them attractive candidates for therapeutic development.
Toxicity and Safety Considerations
While thiols have numerous beneficial applications, certain thiols exhibit toxicity. Low molecular weight thiols, particularly methanethiol, exhibit a pungent odor and can be toxic at high concentrations. Appropriate safety precautions are necessary when handling thiols, including the use of personal protective equipment (PPE) and proper ventilation.
Conclusion: The Versatility of Thiols
Thiols, despite their structural similarity to alcohols, possess remarkably distinct properties and reactivity. Their nucleophilicity, ability to form disulfides, and characteristic odor make them invaluable in diverse applications ranging from biochemistry and medicine to industry. Further research into thiol chemistry is expected to unveil new applications and therapeutic potential, solidifying their significance in various fields. The ongoing study of thiols continues to reveal their multifaceted nature and contributions to scientific advancement. The differences between their sulfur-containing functional group and the hydroxyl group in alcohols open up a fascinating world of unique properties and potential uses. From their pervasive role in biological systems to their industrial applications, thiols remain an essential area of study in chemistry and related fields. Understanding the similarities and differences between thiols and alcohols provides a foundation for further exploration of these important organic molecules.
Latest Posts
Related Post
Thank you for visiting our website which covers about Thiols Have Structures Similar To Alcohols Except That They Contain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.