The Specificity Of Hormone Action Derives From
Holbox
Mar 23, 2025 · 6 min read
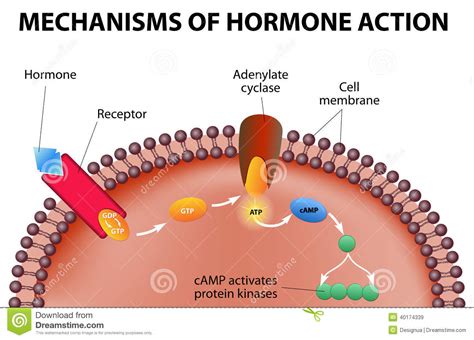
Table of Contents
- The Specificity Of Hormone Action Derives From
- Table of Contents
- The Specificity of Hormone Action Derives From: A Deep Dive into Receptor Binding and Signaling Cascades
- The Crucial Role of Hormone Receptors
- Receptor Types and Their Specificity
- Signal Transduction Cascades: Amplifying and Diversifying Hormonal Signals
- Second Messengers and their Roles
- Cross-talk and Integration of Signals
- Cellular Context: The Influence of Cell Type and Tissue Specificity
- Beyond Receptor Binding: Non-genomic Effects and Membrane-bound Enzymes
- Conclusion: A Complex Interplay of Factors
- Latest Posts
- Latest Posts
- Related Post
The Specificity of Hormone Action Derives From: A Deep Dive into Receptor Binding and Signaling Cascades
Hormones, the body's chemical messengers, orchestrate a vast array of physiological processes, from metabolism and growth to reproduction and mood regulation. The remarkable precision with which hormones exert their effects, influencing specific target cells and eliciting distinct responses, hinges on a sophisticated interplay of factors. This article explores the fundamental mechanisms underlying the specificity of hormone action, focusing on the critical roles of receptor binding, signal transduction pathways, and the intricate cellular context.
The Crucial Role of Hormone Receptors
The specificity of hormone action primarily stems from the interaction between hormones and their cognate receptors. Hormones are not simply broadcast throughout the body, haphazardly affecting any cell they encounter. Instead, they interact with specific receptor proteins, which act as molecular locks that only fit specific hormonal keys. These receptors are typically located on the cell surface or within the cell's interior, depending on the hormone's chemical nature (hydrophilic or hydrophobic).
Receptor Types and Their Specificity
Several key receptor types contribute to hormone-specific action:
-
G protein-coupled receptors (GPCRs): These membrane-bound receptors represent the largest family of cell surface receptors. Upon hormone binding, GPCRs activate intracellular signaling cascades involving G proteins and second messengers, leading to diverse cellular responses. The remarkable diversity of GPCRs and their associated signaling pathways ensures highly specific cellular responses to different hormones. Examples include receptors for adrenaline, glucagon, and many peptide hormones.
-
Receptor tyrosine kinases (RTKs): These transmembrane receptors possess intrinsic tyrosine kinase activity. Hormone binding induces receptor dimerization and autophosphorylation, triggering downstream signaling pathways that often involve MAP kinases and PI3 kinases. These pathways are critically involved in cell growth, differentiation, and survival. Insulin and growth factors utilize RTKs. The unique substrate specificity of each RTK contributes to the specificity of hormonal signaling.
-
Nuclear receptors: These receptors are located within the cell's nucleus or cytoplasm and directly interact with DNA. Steroid hormones, thyroid hormones, and retinoids are examples of ligands that bind to nuclear receptors. Upon binding, these hormone-receptor complexes modulate gene expression, influencing the production of specific proteins. The precise DNA sequences recognized by each nuclear receptor determine the specific genes regulated and, ultimately, the cellular response.
-
Ligand-gated ion channels: These receptors, found on the cell membrane, directly regulate the flow of ions across the membrane upon hormone binding. Neurotransmitters, such as acetylcholine, and some peptide hormones utilize this mechanism. The specific ions affected and the subsequent changes in membrane potential contribute to the precise action of these hormones.
The high degree of specificity inherent in these receptor types arises from the precise three-dimensional structure of the binding site, which only accommodates the correct hormone molecule. Even minor structural differences in the hormone or receptor can significantly alter binding affinity and biological activity. This exquisite molecular recognition mechanism is the cornerstone of hormone specificity.
Signal Transduction Cascades: Amplifying and Diversifying Hormonal Signals
Hormone binding to its receptor initiates a cascade of intracellular events known as signal transduction. These cascades act to amplify the initial hormonal signal, leading to a significant cellular response despite the often low concentration of the circulating hormone. Furthermore, these pathways allow for diversification of hormonal signals, where a single hormone can trigger various responses depending on the cell type and the presence of other signaling molecules.
Second Messengers and their Roles
Second messengers, such as cAMP, IP3, DAG, and calcium ions, play critical roles in signal transduction. These molecules are produced or released upon receptor activation and act to relay the hormonal signal to downstream effector molecules, often enzymes or transcription factors. The specific second messengers generated upon hormone binding are largely determined by the type of receptor and its associated signaling pathways, contributing to the specificity of the cellular response.
Cross-talk and Integration of Signals
Cellular responses are rarely orchestrated by a single hormone acting in isolation. Cells are constantly bombarded by a multitude of signals, and their response reflects the integration of these signals. Cross-talk occurs when different signaling pathways interact, either synergistically or antagonistically. This cross-talk enables a complex and dynamic regulatory system, refining the specificity of hormonal action within the cellular context. For example, the simultaneous presence of two hormones might be necessary to elicit a specific response, or one hormone might inhibit the action of another.
Cellular Context: The Influence of Cell Type and Tissue Specificity
The specificity of hormone action is not solely determined by the hormone-receptor interaction and downstream signaling cascades. The cellular context also plays a crucial role. The same hormone can trigger vastly different responses in different cell types due to variations in:
-
Receptor expression: Cells express specific sets of receptors, which dictates which hormones they can respond to. The number and type of receptors expressed can vary across tissues and even within the same tissue. This differential receptor expression ensures tissue-specific responses to hormones.
-
Signaling molecules: The presence or absence of specific signaling molecules can determine whether or not a cell responds to a given hormone. For example, a cell might lack a particular enzyme necessary for relaying the signal, preventing a downstream response.
-
Gene expression profiles: Cellular responses to hormones are ultimately reflected in altered gene expression patterns. The specific genes expressed in a cell determine which proteins are produced, influencing the cell's response to hormonal signals.
-
Metabolic state: The metabolic status of a cell can also significantly influence its response to hormonal signals. For example, a nutrient-deprived cell might respond differently to insulin than a well-fed cell.
Tissue-specific responses represent another crucial layer of specificity. Different tissues express distinct sets of receptors and signaling molecules, leading to tissue-specific responses to the same hormone. For example, insulin stimulates glucose uptake in muscle and fat tissue but has different effects on the liver.
Beyond Receptor Binding: Non-genomic Effects and Membrane-bound Enzymes
While much emphasis is placed on the role of receptor binding in dictating hormonal specificity, it's crucial to note other mechanisms that contribute to this intricate process:
-
Non-genomic effects: These rapid actions of hormones, which do not involve changes in gene transcription, add another layer of complexity to hormonal signaling. Certain steroid hormones, for example, can directly interact with membrane-bound receptors, leading to rapid changes in intracellular signaling, independent of their classical nuclear receptor mechanisms.
-
Membrane-bound enzymes: The activity of membrane-bound enzymes, which may be directly or indirectly modulated by hormone binding to nearby receptors, contributes to the specificity of hormonal action. These enzymes often catalyze reactions that affect intracellular signaling molecules, propagating the hormone's signal.
Conclusion: A Complex Interplay of Factors
The specificity of hormone action is a multifaceted process stemming from a complex interplay of factors. While the interaction between hormones and their cognate receptors forms the foundation of this specificity, the subsequent signal transduction cascades, the cellular context, the presence of other signaling molecules, and even non-genomic effects all contribute to the precise and diverse responses elicited by hormones throughout the body. Understanding these mechanisms is crucial for comprehending the intricate control of physiological processes and for developing targeted therapeutic interventions for hormonal imbalances and diseases. Further research continually unveils new layers of complexity in this field, highlighting the remarkable precision and sophistication of hormonal signaling. The specificity of hormonal action underscores the body's remarkable capacity for finely tuned regulation and control.
Latest Posts
Latest Posts
-
Mass Of Sulfur In Copper Sulfide
Mar 26, 2025
-
Productivity Is The Amount Of Goods And Services
Mar 26, 2025
-
The Graph Below Shows The Monopolistically Competitive Market For Smartphones
Mar 26, 2025
-
The Norton Introduction To Literature Shorter 14th Edition
Mar 26, 2025
-
A Food Worker Prepares A Raw Fish Fillet For Cooking
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about The Specificity Of Hormone Action Derives From . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
