The Sister Chromatids Are Moving Apart
Holbox
Mar 29, 2025 · 6 min read
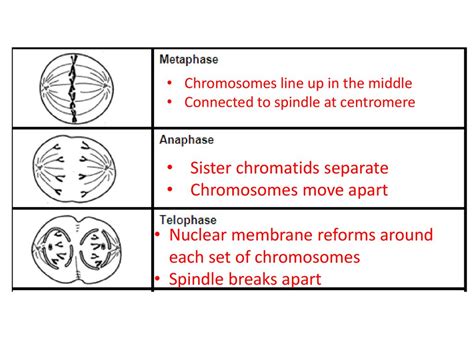
Table of Contents
- The Sister Chromatids Are Moving Apart
- Table of Contents
- The Sister Chromatids Are Moving Apart: A Deep Dive into Anaphase
- Understanding Sister Chromatids and Their Connection
- The Centromere: The Key to Sister Chromatid Cohesion
- The Onset of Anaphase: A Controlled Separation
- Anaphase A: Sister Chromatid Separation
- Anaphase B: Spindle Elongation
- The Significance of the Spindle Assembly Checkpoint (SAC)
- SAC Components and Their Role
- The Anaphase-Promoting Complex/Cyclosome (APC/C): The Master Regulator of Anaphase
- Errors in Anaphase and Their Consequences
- Conclusion: Anaphase—A Crucial Step in Maintaining Life
- Latest Posts
- Latest Posts
- Related Post
The Sister Chromatids Are Moving Apart: A Deep Dive into Anaphase
The cell cycle, a meticulously orchestrated series of events, is fundamental to life itself. Within this cycle, the process of mitosis stands out as a critical stage where a single cell divides into two identical daughter cells. A pivotal moment within mitosis is anaphase, characterized by the dramatic separation of sister chromatids. This article will delve into the intricate mechanisms driving this separation, exploring the molecular players, regulatory processes, and the crucial implications of anaphase for cellular fidelity and organismal health.
Understanding Sister Chromatids and Their Connection
Before diving into the mechanics of anaphase, it's crucial to understand the nature of sister chromatids. During the S phase (synthesis phase) of the cell cycle, each chromosome replicates itself, creating two identical copies called sister chromatids. These chromatids remain tightly bound together at a specialized region called the centromere. This connection is essential, as it ensures that each daughter cell receives a complete and identical set of genetic information.
The Centromere: The Key to Sister Chromatid Cohesion
The centromere is more than just a connection point; it's a complex structure composed of highly repetitive DNA sequences and a variety of proteins. These proteins form a crucial structure called the kinetochore, a protein complex that serves as the attachment site for microtubules, the structural components of the spindle apparatus. The kinetochore's intricate composition plays a critical role in accurately segregating sister chromatids during anaphase. Without proper centromere function, accurate chromosome segregation is impossible, leading to aneuploidy—an abnormal number of chromosomes—in daughter cells. This can have severe consequences, ranging from cell death to the development of cancer.
The Onset of Anaphase: A Controlled Separation
Anaphase, the stage where sister chromatids separate, is not a spontaneous event. It's a tightly regulated process triggered by a series of precisely timed molecular events. This phase is further divided into two sub-stages: anaphase A and anaphase B.
Anaphase A: Sister Chromatid Separation
Anaphase A is marked by the dramatic separation of sister chromatids. This separation is driven by the shortening of kinetochore microtubules, which are attached to the kinetochores of each sister chromatid. The mechanism of microtubule shortening remains a subject of ongoing research, but several models are proposed. One leading hypothesis suggests that microtubules depolymerize at their kinetochore ends, effectively "pulling" the chromatids apart. This process is highly regulated to ensure that sister chromatids separate simultaneously and accurately. Errors in this process can lead to unequal distribution of chromosomes, resulting in aneuploidy.
The Role of Motor Proteins
Motor proteins, such as dynein and kinesin, play crucial roles in anaphase A. Dynein, located at the poles of the spindle, moves along astral microtubules (microtubules radiating outward from the centrosomes) to pull the chromosomes toward the poles. Kinesins, on the other hand, are involved in both chromosome movement and spindle elongation. These motor proteins work in concert to ensure the precise and efficient movement of chromosomes during anaphase A.
Anaphase B: Spindle Elongation
While anaphase A focuses on sister chromatid separation, anaphase B involves the elongation of the entire spindle apparatus. This elongation pushes the separated chromosomes further apart, contributing to the complete separation of the genetic material. Anaphase B is driven by two main mechanisms:
- Sliding of antiparallel microtubules: Microtubules from opposite poles overlap in the spindle midzone. Motor proteins, such as kinesin-5, move along these overlapping microtubules, causing them to slide past each other. This sliding action pushes the poles further apart.
- Pulling forces on astral microtubules: Astral microtubules, extending from the spindle poles to the cell cortex, interact with the cell membrane. Motor proteins, such as dynein, generate pulling forces on these microtubules, effectively pulling the poles apart.
The coordinated actions of anaphase A and anaphase B ensure the complete and accurate segregation of chromosomes to the opposite poles of the dividing cell.
The Significance of the Spindle Assembly Checkpoint (SAC)
The fidelity of chromosome segregation is paramount for the maintenance of genomic integrity. To ensure that all chromosomes are correctly attached to the spindle before anaphase begins, the cell employs a crucial surveillance mechanism: the spindle assembly checkpoint (SAC). The SAC monitors the attachment of kinetochores to microtubules. If any kinetochore is not properly attached, the SAC signals the cell cycle to arrest in metaphase, preventing premature anaphase onset. This prevents the potential for chromosome mis-segregation and the resulting aneuploidy.
SAC Components and Their Role
The SAC involves a complex network of proteins that act as sensors, signaling components, and effectors. Key components include:
- Mad2: Acts as a key sensor of unattached kinetochores.
- BubR1 and Bub1: Contribute to the SAC signal amplification.
- Cdc20: A crucial regulator of anaphase onset. The SAC inhibits Cdc20, preventing the activation of the anaphase-promoting complex/cyclosome (APC/C).
The SAC's intricate mechanism ensures that anaphase initiation is delayed until all chromosomes are correctly attached, preventing errors in chromosome segregation.
The Anaphase-Promoting Complex/Cyclosome (APC/C): The Master Regulator of Anaphase
The APC/C is a ubiquitin ligase, an enzyme that attaches ubiquitin tags to target proteins, marking them for degradation by the proteasome. The APC/C plays a crucial role in triggering anaphase by targeting two key proteins for degradation:
- Securin: Securin holds sister chromatids together by preventing separase activity. APC/C-mediated degradation of securin releases separase, allowing it to cleave cohesins, the proteins that link sister chromatids together.
- Cyclin B: A crucial regulator of the mitotic cyclins. APC/C-mediated degradation of cyclin B triggers the exit from mitosis, allowing the cell to progress into cytokinesis (cell division).
The APC/C's action of targeting Securin and Cyclin B ensures the timely and precise progression of anaphase and the subsequent transition into cytokinesis.
Errors in Anaphase and Their Consequences
Although meticulously regulated, errors can occur during anaphase. These errors can have severe consequences, ranging from cell death to the development of cancer. Common errors include:
- Chromosome mis-segregation: Failure to correctly segregate chromosomes can lead to aneuploidy, an imbalance in the number of chromosomes in daughter cells. Aneuploidy is often associated with developmental abnormalities, cell death, and cancer.
- Lagging chromosomes: Occasionally, chromosomes fail to attach to the spindle properly, causing them to lag behind during anaphase. These lagging chromosomes can be lost during cell division, leading to aneuploidy.
- Merotelic attachment: A single chromosome is attached to microtubules from both spindle poles. This type of mis-attachment can prevent proper segregation during anaphase.
The high fidelity of anaphase is critical for maintaining genomic stability, ensuring that daughter cells receive the correct number of chromosomes and preventing the development of genetic diseases.
Conclusion: Anaphase—A Crucial Step in Maintaining Life
The separation of sister chromatids during anaphase is a remarkable feat of cellular machinery. This tightly regulated process, governed by a complex interplay of molecular players and regulatory mechanisms, ensures the faithful transmission of genetic information from one generation of cells to the next. The consequences of errors in anaphase are severe, highlighting the fundamental importance of this stage in maintaining genomic stability and cellular health. Ongoing research continues to unravel the intricate details of anaphase, leading to a deeper understanding of this crucial stage in the cell cycle and its implications for human health and disease. The more we understand the precise mechanics and regulation of anaphase, the better equipped we will be to address and potentially prevent diseases stemming from errors in chromosome segregation.
Latest Posts
Latest Posts
-
What Does Being A Manager Offer To An Employee
Apr 01, 2025
-
The Function Requires That Management Evaluate Operations Against Some Norm
Apr 01, 2025
-
Select The Two Primary Characteristics That Define Advertising
Apr 01, 2025
-
Trade Can Make Everyone Better Off Because It
Apr 01, 2025
-
Which Country Is Credited For The Birth Of Management
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Sister Chromatids Are Moving Apart . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
