The Phrase Like Dissolves Like Refers To
Holbox
Mar 17, 2025 · 6 min read
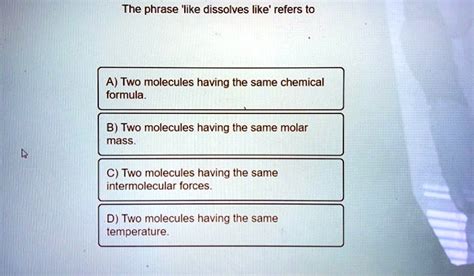
Table of Contents
The Phrase "Like Dissolves Like": A Deep Dive into Solubility
The phrase "like dissolves like" is a fundamental principle in chemistry, governing the solubility of substances. It essentially states that substances with similar intermolecular forces tend to be soluble in each other. Understanding this principle is crucial for various applications, from pharmaceutical development to environmental science. This article will delve into the intricacies of "like dissolves like," exploring its underlying mechanisms, exceptions, and practical implications.
Understanding Intermolecular Forces: The Key to Solubility
Before delving into the "like dissolves like" principle, it's essential to understand the role of intermolecular forces. These are the attractive forces between molecules, and their strength dictates the physical properties of a substance, including its boiling point, melting point, and, critically, its solubility. The major types of intermolecular forces include:
1. London Dispersion Forces (LDFs):
These are the weakest intermolecular forces and are present in all molecules. They arise from temporary fluctuations in electron distribution, creating temporary dipoles that induce dipoles in neighboring molecules. The strength of LDFs increases with the size and surface area of the molecule. Larger molecules with more electrons exhibit stronger LDFs.
2. Dipole-Dipole Interactions:
These forces occur between polar molecules – molecules with a permanent dipole moment due to a difference in electronegativity between atoms. The positive end of one polar molecule is attracted to the negative end of another. Dipole-dipole interactions are stronger than LDFs.
3. Hydrogen Bonding:
A special type of dipole-dipole interaction, hydrogen bonding occurs when a hydrogen atom bonded to a highly electronegative atom (oxygen, nitrogen, or fluorine) is attracted to a lone pair of electrons on another electronegative atom. Hydrogen bonds are relatively strong intermolecular forces and significantly influence the properties of many substances.
4. Ion-Dipole Interactions:
These interactions occur between ions and polar molecules. The positive or negative ion is attracted to the oppositely charged end of the polar molecule. Ion-dipole interactions are strong and play a significant role in the solubility of ionic compounds in polar solvents.
"Like Dissolves Like" in Action: Examples and Explanations
The "like dissolves like" rule essentially means that polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes. Let's explore this with some examples:
1. Polar Solvents and Polar Solutes:
Water (H₂O) is a classic example of a polar solvent. Its molecule has a bent shape, leading to a significant dipole moment. Water readily dissolves many polar solutes, such as sugar (sucrose) and salt (sodium chloride, NaCl). This is because the polar water molecules can interact strongly with the polar functional groups in sugar (hydroxyl groups, -OH) and the ions in salt through ion-dipole interactions. The strong interactions between the solvent and solute molecules overcome the intermolecular forces within the solute, allowing it to dissolve.
Example: Sugar dissolving in water. The hydroxyl groups in sugar engage in hydrogen bonding with water molecules, facilitating dissolution.
2. Nonpolar Solvents and Nonpolar Solutes:
Nonpolar solvents, such as hexane (C₆H₁₄) and benzene (C₆H₆), lack a significant dipole moment. They primarily interact through weak London Dispersion Forces. These solvents effectively dissolve nonpolar solutes, such as oils and fats, which are also primarily held together by LDFs. The similar intermolecular forces allow for relatively easy mixing.
Example: Oil dissolving in gasoline. Both oil and gasoline are primarily composed of nonpolar hydrocarbon molecules, interacting through LDFs.
3. Ionic Compounds in Polar Solvents:
Ionic compounds, such as NaCl, are composed of ions held together by strong electrostatic forces. However, they are often soluble in polar solvents like water. The polar water molecules surround the ions, effectively shielding them from each other and reducing the strong electrostatic attractions. This process, known as solvation or hydration (when water is the solvent), allows the ionic compound to dissolve.
Example: Salt dissolving in water. Water molecules surround the Na⁺ and Cl⁻ ions, weakening the ionic bonds and allowing dissolution.
Exceptions to the Rule: When "Like Dissolves Like" Doesn't Apply
While "like dissolves like" is a valuable guideline, it's not an absolute rule. Several exceptions exist:
1. Small Nonpolar Molecules in Water:
Some small nonpolar molecules, such as oxygen (O₂) and carbon dioxide (CO₂), possess some solubility in water despite being nonpolar. This limited solubility is attributed to weak interactions between the nonpolar molecule and the water molecules, specifically London Dispersion Forces.
2. The Role of Temperature and Pressure:
Temperature and pressure significantly influence solubility. Increasing the temperature generally increases the solubility of solids and gases in liquids, while increasing the pressure increases the solubility of gases. These factors can sometimes override the "like dissolves like" principle.
3. Formation of Complexes:
The formation of complexes between the solute and solvent can enhance solubility, even if the solute and solvent have significantly different polarities. These complexes often involve coordinate covalent bonds.
4. Hydrogen Bonding and Surfactants:
Surfactants are amphiphilic molecules with both polar and nonpolar regions. Their unique structure allows them to dissolve both polar and nonpolar substances, acting as a bridge between the two. This is a significant exception, often defying the simple "like dissolves like" principle. Their ability to lower surface tension facilitates the mixing of otherwise immiscible liquids.
Applications of "Like Dissolves Like": From Everyday Life to Advanced Technologies
The "like dissolves like" principle has far-reaching implications across various fields:
1. Pharmaceutical Development:
Understanding solubility is critical in drug formulation. Drugs must dissolve in the body's fluids to be absorbed and exert their therapeutic effect. Pharmaceutical scientists carefully choose solvents and excipients to ensure adequate drug solubility and bioavailability.
2. Environmental Science:
Solubility plays a vital role in understanding pollutant behavior in the environment. The solubility of pollutants in water determines their transport and fate in aquatic systems. Nonpolar pollutants, for example, tend to accumulate in fatty tissues of organisms, leading to bioaccumulation and biomagnification.
3. Chemical Engineering:
Solubility governs various chemical processes, including extraction, purification, and crystallization. Engineers use solvents selectively to separate and purify components of a mixture based on their solubility differences.
4. Cosmetics and Personal Care:
Many cosmetic products rely on the principle of "like dissolves like." For example, oil-based cleansers effectively remove makeup and other oil-based substances because they dissolve them through similar intermolecular forces.
5. Food Science:
Solubility impacts many food-related processes. The solubility of different ingredients determines their interaction and behavior during food processing and storage. Understanding solubility is essential for creating stable and appealing food products.
Conclusion: A Foundation for Understanding Chemical Interactions
The "like dissolves like" principle provides a fundamental framework for understanding solubility and its implications. While not a universally applicable rule without exceptions, it serves as an excellent starting point for predicting the solubility of substances. By understanding the interplay of intermolecular forces and the various factors influencing solubility, we gain valuable insight into a wide range of chemical phenomena and applications across many scientific and technological domains. Further exploration into the intricacies of various solvents, solutes, and their interactions will continue to refine our understanding of this crucial chemical concept. Remember, while the rule serves as a useful guideline, always consider the nuanced exceptions and contributing factors that may influence the solubility of any given substance.
Latest Posts
Latest Posts
-
Correctly Label The Following Parts Of The Adrenal Gland
Mar 17, 2025
-
A 2019 Study Published In Nature Ecology
Mar 17, 2025
-
Your Leader Asks You To Help Unload And Organize Merchandise
Mar 17, 2025
-
Name The Membranous Encasement Surrounding The Brain
Mar 17, 2025
-
What Is The Definition For The Protection Mission Area
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Phrase Like Dissolves Like Refers To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
