The Diels Alder Reaction Is A Concerted Reaction. Define Concerted.
Holbox
Mar 20, 2025 · 6 min read
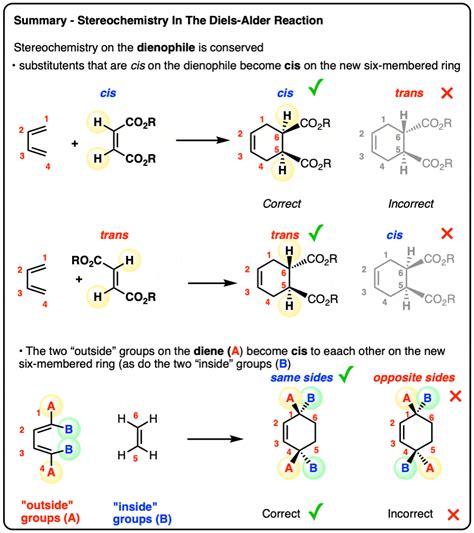
Table of Contents
The Diels-Alder Reaction: A Concerted Cycloaddition
The Diels-Alder reaction, a cornerstone of organic chemistry, stands as a prime example of a concerted pericyclic reaction. Understanding its concerted nature is key to grasping its mechanism, regio- and stereoselectivity, and synthetic utility. But what exactly does "concerted" mean in this context? This article delves deep into the definition of concerted reactions, explores the intricacies of the Diels-Alder reaction, and examines its significance in organic synthesis.
Defining "Concerted" in Chemical Reactions
In the realm of chemical reactions, the term "concerted" signifies a reaction mechanism where bond breaking and bond formation occur simultaneously, without the formation of any intermediates. This contrasts sharply with stepwise reactions, which proceed through distinct, isolable intermediates. In a concerted reaction, the transition state represents the highest energy point along the reaction coordinate, encompassing both bond breaking and bond making. There's no discrete stage where the molecule exists as a distinct intermediate with a different structure. The process is a single, continuous transformation.
The concerted nature of a reaction has significant implications for its stereochemistry and reaction kinetics. Because bond breaking and bond making are synchronous, the stereochemical relationships in the reactants are often directly translated into the products. This is a crucial aspect of the Diels-Alder reaction's predictability and utility. The absence of intermediates also simplifies the reaction mechanism, making it often easier to predict the outcome and control reaction conditions.
The Diels-Alder Reaction: A Paradigm of Concertedness
The Diels-Alder reaction is a [4+2] cycloaddition, meaning a four-carbon atom component (the diene) reacts with a two-carbon atom component (the dienophile) to form a six-membered ring. This transformation is famously concerted, proceeding through a single, cyclic transition state without the involvement of any intermediates.
The Mechanism: The reaction begins with the approach of the diene and dienophile. The diene must adopt the s-cis conformation to allow for the cycloaddition. The orbitals of the diene and dienophile interact in a suprafacial manner, meaning that the additions occur from the same side of each reactant. This interaction leads to the simultaneous formation of two new sigma bonds and the breaking of one pi bond in the diene and one pi bond in the dienophile. All these processes happen synchronously within the single transition state. The resulting cyclohexene derivative inherits the stereochemical features of both reactants.
Visualizing the Concerted Mechanism
Imagine the diene and dienophile approaching each other. The pi orbitals of the diene and dienophile overlap in a way that facilitates simultaneous bond formation. There is no intermediate carbocation or carbanion, no discrete step where the reaction pauses. It's a continuous, smooth flow of electron density from the diene to the dienophile, resulting in the cyclic product.
Evidence for the Concerted Mechanism
Several lines of evidence strongly support the concerted mechanism of the Diels-Alder reaction:
-
Stereospecificity: The stereochemistry of the reactants is preserved in the product. A cis dienophile yields a cis substituted cyclohexene, while a trans dienophile gives a trans substituted cyclohexene. This strict stereochemical control is inconsistent with a stepwise mechanism that would allow for bond rotation and isomerization.
-
Kinetic Isotope Effects: Studies using isotopically labeled reactants show that the rate of the reaction is not significantly affected by isotopic substitution at the reacting carbons. This is indicative of a concerted mechanism, as a stepwise mechanism would involve breaking bonds, with isotopic substitution expected to show a clear kinetic difference.
-
Lack of Intermediates: Despite extensive efforts, no intermediates have ever been isolated or detected during the Diels-Alder reaction. This observation provides strong support for a concerted mechanism.
-
Theoretical Calculations: Advanced computational chemistry techniques have provided detailed insights into the Diels-Alder reaction's transition state. These calculations strongly support the concerted nature of the reaction, with the transition state showing synchronous bond formation and bond breaking.
Factors Influencing the Diels-Alder Reaction
Several factors influence the rate and selectivity of the Diels-Alder reaction:
-
Electron Demand: The reaction is favored by electron-rich dienes and electron-deficient dienophiles (or vice versa – inverse electron demand Diels-Alder reaction). Electron-donating groups on the diene and electron-withdrawing groups on the dienophile accelerate the reaction.
-
Steric Effects: Bulky substituents on the diene or dienophile can hinder the approach of the reactants, reducing the reaction rate.
-
Solvent Effects: The reaction is often accelerated in polar solvents, which stabilize the transition state.
-
Temperature: The reaction rate typically increases with temperature. Higher temperatures can promote the reaction but may also lead to side reactions.
-
Pressure: High pressure also favours Diels-Alder reactions.
Regioselectivity and Stereoselectivity in Diels-Alder Reactions
The Diels-Alder reaction exhibits remarkable regio- and stereoselectivity. This means that the reaction favors the formation of a specific regioisomer (different arrangement of substituents) and stereoisomer (different spatial arrangement of atoms) among many possibilities. These selectivities are governed by orbital interactions and steric considerations.
Regioselectivity
Regioselectivity is predicted by considering the relative electron-donating and electron-withdrawing capabilities of the substituents on the diene and dienophile. The most electron-rich carbon of the diene will preferentially bond with the most electron-deficient carbon of the dienophile, following the principle of maximum orbital overlap. This principle maximizes the stability of the transition state.
Stereoselectivity
Stereoselectivity refers to the preferential formation of one stereoisomer over others. In Diels-Alder reactions, the endo approach is favored over the exo approach, leading to the formation of the endo product predominantly. The endo product refers to the stereochemistry where the substituents on the dienophile are oriented towards the newly formed bridgehead carbons of the cyclohexene ring. The endo selectivity results from secondary orbital interactions between the diene and the dienophile, which stabilize the transition state leading to the endo product. However, steric effects can sometimes outweigh this endo selectivity.
Synthetic Applications of the Diels-Alder Reaction
The Diels-Alder reaction is a powerful tool in organic synthesis due to its:
-
Atom Economy: The reaction creates new C-C bonds in a highly efficient manner, incorporating almost all atoms of the starting materials into the product.
-
Stereoselectivity: The reaction often provides highly stereoselective access to complex cyclohexene derivatives, which are important structural motifs in many natural products and pharmaceuticals.
-
Versatility: The reaction can be applied to a vast array of dienes and dienophiles, offering a wide range of synthetic possibilities. Modifications such as inverse electron demand Diels-Alder reactions further expand its scope and versatility.
The reaction plays a crucial role in constructing various cyclic systems, including those found in steroids, terpenes, and other biologically active compounds. It's a cornerstone reaction in the synthesis of numerous natural products and pharmaceuticals.
Conclusion: The Concerted Power of the Diels-Alder Reaction
The Diels-Alder reaction's concerted mechanism is the key to its efficiency, regio- and stereoselectivity, and widespread applications. This inherent efficiency makes it an indispensable tool for organic chemists, contributing significantly to the synthesis of complex molecules with remarkable precision and control. The simultaneous bond formation and bond breaking represent the elegant simplicity and power of this pericyclic reaction, cementing its place as one of the most important and well-studied reactions in organic chemistry. Further research continues to unravel the finer details of the reaction and expand its synthetic potential. The exploration of novel dienes and dienophiles, combined with sophisticated reaction optimization techniques, promises to unveil new facets of this powerful and versatile reaction, keeping its significance in synthetic organic chemistry strong for years to come.
Latest Posts
Latest Posts
-
To Avoid Fatigue When Should Team Roles
Mar 20, 2025
-
Which Sentence Uses The Underlined Word Correctly
Mar 20, 2025
-
The Great Compromise Did All Of The Following Except
Mar 20, 2025
-
Altering The Three Dimensional Structure Of An Enzyme Might
Mar 20, 2025
-
According To Ich E6 An Audit Is Defined As
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about The Diels Alder Reaction Is A Concerted Reaction. Define Concerted. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
