Spell Out The Full Name Of The Compound
Holbox
Mar 16, 2025 · 5 min read
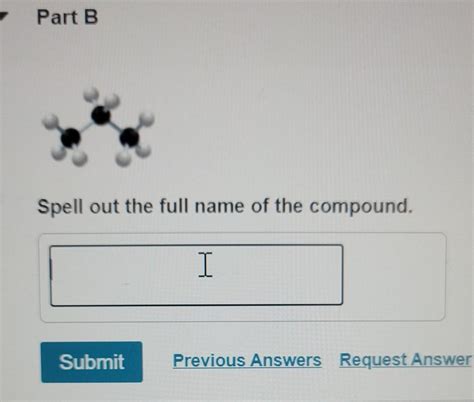
Table of Contents
Spell Out the Full Name of the Compound: A Deep Dive into Chemical Nomenclature
Chemical nomenclature, the system of naming chemical compounds, is a crucial aspect of chemistry. It allows chemists worldwide to communicate unambiguously about specific substances. Understanding how to name compounds, and conversely, how to determine the structure of a compound from its name, is fundamental to anyone working in the chemical sciences. This article will delve into the intricacies of chemical nomenclature, focusing on how to spell out the full name of a compound, covering various types of compounds and their associated naming conventions.
The Importance of Accurate Chemical Naming
The precise spelling and complete name of a chemical compound are not mere formalities; they are critical for safety, research, and industrial applications. A small error in spelling can lead to confusion, potentially causing serious consequences, especially in contexts like medicine, manufacturing, and environmental science. Consider the difference between a compound with a similar name but different structural arrangement: a slight variation can drastically alter its properties and effects.
Ambiguity breeds errors. Abbreviations or shortened names, while convenient, can easily lead to misunderstandings. The full systematic name eliminates ambiguity and ensures clarity in all communication related to the compound.
Different Types of Compounds and Their Naming Conventions
Several categories of chemical compounds exist, each with its specific naming rules. Let's explore some of the most common ones:
1. Ionic Compounds (Salts)
Ionic compounds are formed from the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). Their names generally follow this pattern:
- Cation name + Anion name
For example:
- NaCl: Sodium Chloride (Sodium cation + Chloride anion)
- K₂SO₄: Potassium Sulfate (Potassium cation + Sulfate anion)
- Ca(NO₃)₂: Calcium Nitrate (Calcium cation + Nitrate anion)
Polyatomic ions: When dealing with polyatomic ions (ions composed of multiple atoms), their full names must be used. For instance, "sulfate" (SO₄²⁻) must be written in full, rather than abbreviated as "SO₄".
Transition metals: Transition metals can have variable oxidation states. The oxidation state (charge) of the cation must be indicated using Roman numerals in parentheses after the cation name. For example:
- FeCl₂: Iron(II) Chloride (Iron with a +2 charge)
- FeCl₃: Iron(III) Chloride (Iron with a +3 charge)
2. Covalent Compounds (Molecular Compounds)
Covalent compounds are formed when atoms share electrons. Their naming system differs from that of ionic compounds:
- Prefix + Name of less electronegative element + Prefix + Name of more electronegative element + "-ide"
The prefixes indicate the number of atoms of each element present:
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
For example:
- CO₂: Carbon Dioxide (One carbon atom, two oxygen atoms)
- N₂O₄: Dinitrogen Tetroxide (Two nitrogen atoms, four oxygen atoms)
- PCl₅: Phosphorus Pentachloride (One phosphorus atom, five chlorine atoms)
Note that the prefix "mono-" is often omitted for the first element unless it is needed for clarity.
3. Acids
Acids are compounds that donate protons (H⁺) in solution. Their naming conventions depend on whether they are binary acids (containing hydrogen and one other element) or oxyacids (containing hydrogen, oxygen, and another element).
-
Binary Acids: The name begins with the prefix "hydro-" followed by the root name of the nonmetal element, then "-ic acid". For example:
- HCl: Hydrochloric acid
- HBr: Hydrobromic acid
- HI: Hydroiodic acid
-
Oxyacids: The naming of oxyacids is more complex and depends on the oxidation state of the nonmetal element. The root name is derived from the nonmetal, and the suffixes "-ous acid" or "-ic acid" are used to distinguish between different oxidation states. For instance:
- HNO₂: Nitrous acid
- HNO₃: Nitric acid
- H₂SO₃: Sulfurous acid
- H₂SO₄: Sulfuric acid
4. Organic Compounds
Organic compounds are carbon-based compounds, and their naming system (IUPAC nomenclature) is significantly more complex than that of inorganic compounds. It involves identifying the longest carbon chain, assigning numbers to the carbons, identifying functional groups, and applying specific rules for branching and multiple functional groups. This requires extensive study and is beyond the scope of this introductory discussion. However, the principle of using the complete and unambiguous name remains paramount.
Practical Applications and Examples
Let's consider some real-world scenarios illustrating the importance of fully spelling out compound names:
-
Pharmaceuticals: The precise naming of pharmaceutical compounds is critical for accurate dosages and to avoid medication errors. A slight misspelling could lead to administering the wrong drug, resulting in severe adverse effects or even death.
-
Industrial Chemistry: In manufacturing processes, using the correct name ensures that the appropriate raw materials are used, and the desired product is synthesized. Misidentification of a chemical can lead to costly production errors and even safety hazards.
-
Environmental Science: Accurate identification of pollutants and contaminants in environmental samples is essential for monitoring and remediation efforts. Using the full chemical name ensures accurate data analysis and interpretation.
-
Research and Development: In scientific publications and research reports, accurate chemical nomenclature is vital for reproducibility and preventing misinterpretations of research findings. Using complete names ensures that others can replicate experiments accurately.
Beyond the Basics: Advanced Nomenclature
The systems described above cover basic nomenclature. More complex compounds, such as coordination compounds, organometallic compounds, and polymers, require even more intricate naming conventions involving ligands, coordination numbers, and polymer structures. These conventions often use a combination of prefixes, suffixes, and numerical indicators to specify the exact composition and structure of the molecule. Specialized literature and advanced chemistry textbooks delve into these complexities.
Conclusion: The Power of Precision in Chemical Naming
Spelling out the full name of a compound is not merely a matter of following rules; it's a fundamental principle that underpins the safety, accuracy, and efficacy of countless processes across various fields. The systematic naming conventions, while sometimes seemingly complex, are designed to eliminate ambiguity and facilitate clear communication among chemists and scientists globally. Embracing the precision inherent in chemical nomenclature safeguards against errors and ensures the integrity of scientific research, industrial applications, and, critically, human safety. Always prioritize the use of the complete systematic name to avoid confusion and maintain the highest standards of accuracy in all chemical-related contexts. This commitment to precision is the bedrock of reliable scientific progress and responsible industrial practice.
Latest Posts
Latest Posts
-
El Hijo De Mi Padre Es Mi
Mar 16, 2025
-
Which One Of The Following Statements Is True
Mar 16, 2025
-
Which Is Not A N Expense Account
Mar 16, 2025
-
In C3 Plants The Conservation Of Water Promotes
Mar 16, 2025
-
Food Preservation Does All Of The Following Except
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Spell Out The Full Name Of The Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
