Several Reagents And Several Organic Structures
Holbox
Mar 30, 2025 · 6 min read
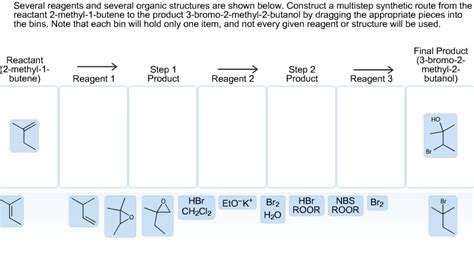
Table of Contents
- Several Reagents And Several Organic Structures
- Table of Contents
- Several Reagents and Several Organic Structures: A Deep Dive into Organic Chemistry Reactions
- Common Reagents and Their Reactivity
- 1. Grignard Reagents (RMgX)
- 2. Organolithium Reagents (RLi)
- 3. Lithium Aluminum Hydride (LiAlH₄)
- 4. Sodium Borohydride (NaBH₄)
- 5. Osmium Tetroxide (OsO₄)
- Diverse Organic Structures and Their Transformations
- 1. Aldehydes and Ketones
- 2. Carboxylic Acids and Esters
- 3. Alkenes
- 4. Alkynes
- 5. Aromatic Compounds
- Advanced Applications and Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Several Reagents and Several Organic Structures: A Deep Dive into Organic Chemistry Reactions
Organic chemistry is a vast and intricate field, revolving around the study of carbon-containing compounds and their reactions. Understanding the behavior of various reagents and their interactions with different organic structures is fundamental to mastering this discipline. This comprehensive article explores several key reagents, detailing their functionalities, reaction mechanisms, and applications in transforming various organic structures. We'll delve into specific examples, highlighting the versatility and importance of these reagents in organic synthesis.
Common Reagents and Their Reactivity
Organic synthesis hinges on the judicious choice of reagents to achieve specific transformations. Let's examine some prominent reagents and their applications:
1. Grignard Reagents (RMgX)
Grignard reagents, organomagnesium halides, are powerful nucleophiles widely used in the formation of carbon-carbon bonds. The carbon atom bonded to magnesium bears a partial negative charge, making it highly reactive towards electrophilic centers.
Reactions:
- Addition to Carbonyl Compounds: Grignard reagents readily add to aldehydes and ketones, forming alcohols. The reaction proceeds via nucleophilic attack of the Grignard reagent on the carbonyl carbon, followed by protonation. For instance, the reaction of methylmagnesium bromide (CH₃MgBr) with formaldehyde (HCHO) yields methanol (CH₃OH).
- Reaction with Esters and Carboxylic Acids: Reaction with esters leads to tertiary alcohols, while reaction with carboxylic acids results in secondary alcohols.
- Formation of Carbon-Carbon Bonds: Grignard reagents can react with epoxides, opening the ring and creating new carbon-carbon bonds.
Limitations: Grignard reagents are sensitive to moisture and react vigorously with protic solvents like water and alcohols. They require anhydrous conditions for successful reactions.
2. Organolithium Reagents (RLi)
Similar to Grignard reagents, organolithium reagents are strong nucleophiles and bases. However, they are generally more reactive than Grignard reagents due to the greater polarity of the carbon-lithium bond.
Reactions:
- Addition to Carbonyl Compounds: Organolithiums also add to carbonyl compounds, producing alcohols. The reactivity is often higher than that of Grignard reagents, potentially leading to over-reaction in some cases.
- Metal-Halogen Exchange: Organolithium reagents can undergo metal-halogen exchange with alkyl halides, providing a route for the synthesis of new organolithiums.
- Deprotonation: Their strong basicity allows them to deprotonate acidic protons, such as those in terminal alkynes or activated aromatic compounds.
Limitations: Similar to Grignard reagents, organolithiums are extremely sensitive to moisture and air, requiring rigorous anhydrous conditions.
3. Lithium Aluminum Hydride (LiAlH₄)
Lithium aluminum hydride (LiAlH₄) is a powerful reducing agent commonly used to reduce a variety of functional groups. It's a strong nucleophile and a strong base.
Reactions:
- Reduction of Aldehydes and Ketones: LiAlH₄ readily reduces aldehydes and ketones to primary and secondary alcohols, respectively.
- Reduction of Esters and Carboxylic Acids: It reduces esters to primary alcohols and carboxylic acids to primary alcohols.
- Reduction of Amides and Nitriles: It converts amides to amines and nitriles to primary amines.
Limitations: LiAlH₄ is highly reactive with water and other protic solvents. Reactions must be carried out under anhydrous conditions. It's a very strong reducing agent, and its reactivity needs to be carefully controlled to avoid over-reduction.
4. Sodium Borohydride (NaBH₄)
Sodium borohydride (NaBH₄) is a milder reducing agent compared to LiAlH₄. It's commonly used for the selective reduction of aldehydes and ketones to alcohols. It is less reactive towards esters and carboxylic acids.
Reactions:
- Reduction of Aldehydes and Ketones: NaBH₄ effectively reduces aldehydes and ketones to alcohols.
- Less Reactive towards Other Functional Groups: Its milder nature makes it useful for selective reductions in the presence of other reducible groups.
Limitations: NaBH₄ is less reactive than LiAlH₄ and may not reduce certain functional groups, such as esters and carboxylic acids, effectively.
5. Osmium Tetroxide (OsO₄)
Osmium tetroxide (OsO₄) is a powerful oxidizing agent used for the syn-dihydroxylation of alkenes. It adds two hydroxyl groups across the double bond with syn stereochemistry.
Reactions:
- Syn-dihydroxylation of Alkenes: OsO₄ reacts with alkenes to form vicinal diols. The reaction proceeds via a cyclic osmate ester intermediate.
Limitations: OsO₄ is highly toxic and expensive. The reaction often requires a co-oxidant, such as N-methylmorpholine N-oxide (NMO), to regenerate the OsO₄ catalyst.
Diverse Organic Structures and Their Transformations
Now, let's examine how these reagents interact with different organic structures:
1. Aldehydes and Ketones
Aldehydes and ketones, characterized by their carbonyl group (C=O), undergo various reactions with the reagents discussed above.
- Grignard and Organolithium Reagents: These reagents add to the carbonyl carbon, yielding alcohols.
- Lithium Aluminum Hydride and Sodium Borohydride: These reducing agents convert aldehydes and ketones to alcohols.
2. Carboxylic Acids and Esters
Carboxylic acids and esters also react with several of the above reagents.
- Grignard and Organolithium Reagents: These reagents react with esters to yield tertiary alcohols and with carboxylic acids (after initial deprotonation) leading to secondary alcohols.
- Lithium Aluminum Hydride: This strong reducing agent converts carboxylic acids and esters to primary alcohols. NaBH4 typically doesn't reduce these functional groups effectively.
3. Alkenes
Alkenes, containing a carbon-carbon double bond, are susceptible to various transformations.
- Osmium Tetroxide: This reagent adds hydroxyl groups across the double bond, resulting in vicinal diols. This reaction is stereospecific, affording syn addition.
- Epoxidation: Peroxyacids (e.g., mCPBA) convert alkenes to epoxides.
4. Alkynes
Alkynes, featuring a carbon-carbon triple bond, also undergo unique reactions.
- Hydrogenation: Hydrogenation using catalysts such as platinum or palladium reduces alkynes to alkenes or alkanes, depending on the reaction conditions.
- Addition Reactions: Alkynes can undergo addition reactions with halogens, hydrogen halides, and water.
5. Aromatic Compounds
Aromatic compounds, characterized by their delocalized pi electron system, show distinct reactivity.
- Electrophilic Aromatic Substitution: Aromatic compounds undergo electrophilic aromatic substitution reactions, where an electrophile substitutes a hydrogen atom on the aromatic ring.
- Grignard/Organolithium Reactions: Organometallic reagents can react with aryl halides under specific conditions.
Advanced Applications and Considerations
The application of these reagents extends far beyond simple transformations. They are crucial components in complex multi-step organic syntheses, enabling the construction of intricate molecules with desired functionalities and stereochemistry.
Protecting Groups: In many syntheses, protecting groups are necessary to selectively modify specific functional groups while leaving others intact. For instance, protecting alcohols as silyl ethers prevents their reaction with strong reducing agents.
Stereochemistry: Many reactions with these reagents influence the stereochemistry of the product. Understanding stereochemical aspects is crucial for designing syntheses that lead to specific stereoisomers.
Reaction Optimization: Reaction conditions, such as solvent, temperature, and concentration, significantly impact reaction yields and selectivity. Optimization is vital for maximizing the efficiency of organic synthesis.
Green Chemistry: The increasing emphasis on green chemistry promotes the development of environmentally benign reagents and reaction conditions, minimizing waste and toxicity.
Conclusion
The reagents and organic structures discussed here represent a small fraction of the vast landscape of organic chemistry. Mastering the properties and reactivity of these key components is fundamental to understanding and conducting organic synthesis. The ability to predict the outcomes of reactions based on the structures of reactants and reagents is essential for designing and executing efficient and effective synthetic strategies. Further exploration into specialized reagents and reaction mechanisms is encouraged to deepen one's understanding of this multifaceted and fascinating field. This article provides a foundation for further learning and exploration in the exciting world of organic chemistry. Continuous learning and practical application are key to becoming proficient in this complex and rewarding field.
Latest Posts
Latest Posts
-
A Local School Administrator Observes An Increase
Apr 02, 2025
-
Noticing That You Have Difficulty Concentrating
Apr 02, 2025
-
Which Of The Following Correctly Explains The Actions An Agent
Apr 02, 2025
-
What Is The Common Name Of The Following Compound
Apr 02, 2025
-
Companies Use Accelerated Depreciation For Tax Purposes Because
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Several Reagents And Several Organic Structures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
