Select The Sketches Of A 3d Orbital.
Holbox
Mar 29, 2025 · 6 min read
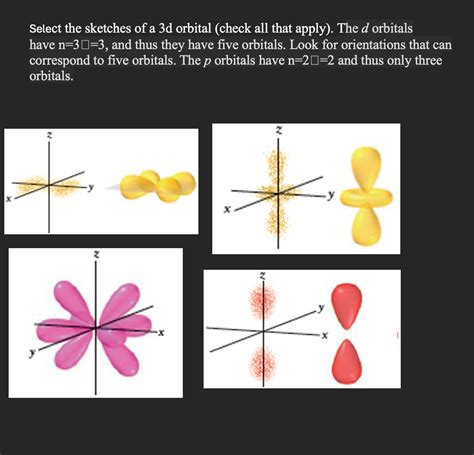
Table of Contents
- Select The Sketches Of A 3d Orbital.
- Table of Contents
- Selecting the Sketches of a 3D Orbital: A Comprehensive Guide
- The Quantum Mechanical Model and Atomic Orbitals
- Common Atomic Orbital Sketches: A Visual Guide
- s Orbitals (l = 0)
- p Orbitals (l = 1)
- d Orbitals (l = 2)
- f Orbitals (l = 3) and beyond
- Selecting Appropriate Sketches: Context is Key
- Interpreting Orbital Sketches: Common Pitfalls
- Advanced Concepts and Applications
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Selecting the Sketches of a 3D Orbital: A Comprehensive Guide
Understanding the shapes and orientations of atomic orbitals is fundamental to grasping the behavior of atoms and molecules. While the exact mathematical description of an atomic orbital is complex, visual representations – sketches – provide an accessible way to comprehend their key features. This article delves into the nuances of selecting and interpreting sketches of 3D orbitals, focusing on the common types encountered in chemistry and physics.
The Quantum Mechanical Model and Atomic Orbitals
Before diving into sketching, let's briefly recap the foundation. The quantum mechanical model describes electrons not as particles orbiting the nucleus in neat, defined paths (like planets around the sun), but as existing in regions of space with varying probabilities of finding the electron. These regions are called atomic orbitals.
Each atomic orbital is characterized by a set of quantum numbers:
- Principal quantum number (n): Determines the energy level and size of the orbital. Larger n values correspond to higher energy and larger orbitals. n can be any positive integer (1, 2, 3, ...).
- Azimuthal quantum number (l): Determines the shape of the orbital and its angular momentum. l can range from 0 to n - 1. l = 0 represents an s orbital, l = 1 represents a p orbital, l = 2 represents a d orbital, and so on.
- Magnetic quantum number (ml): Determines the spatial orientation of the orbital. ml can range from -l to +l, including 0. For p orbitals (l = 1), there are three possible orientations (ml = -1, 0, +1), often denoted as px, py, and pz. For d orbitals (l = 2), there are five orientations.
- Spin quantum number (ms): Describes the intrinsic angular momentum of the electron (spin up or spin down). This isn't directly related to the shape of the orbital but is crucial for understanding electron configuration.
Common Atomic Orbital Sketches: A Visual Guide
The sketches we use are simplified representations, focusing on the regions of space where the probability of finding an electron is highest. These are often depicted as boundary surfaces, encompassing a certain percentage (usually 90%) of the electron density.
s Orbitals (l = 0)
-
1s orbital: Represented as a sphere centered on the nucleus. The electron density is highest at the nucleus and decreases as you move away. The sketch is a simple circle in 2D, representing a cross-section of the sphere.
-
2s orbital: Also spherical, but larger than the 1s orbital. It exhibits a radial node—a spherical surface where the probability of finding the electron is zero. The sketch shows two concentric spheres (or circles in 2D), representing the regions of high electron density separated by the node. Higher n s-orbitals follow a similar pattern with increasing number of nodes.
p Orbitals (l = 1)
-
2p orbitals (px, py, pz): Each 2p orbital has two lobes of electron density on opposite sides of the nucleus, separated by a nodal plane (a plane where the electron probability is zero). The three p orbitals are oriented along the x, y, and z axes, respectively. Sketches often use a dumbbell shape to represent the lobes. Remember that these lobes are three-dimensional, extending into space.
-
Higher n p orbitals: Similar dumbbell shapes, but with additional radial nodes.
d Orbitals (l = 2)
-
3d orbitals: There are five 3d orbitals with more complex shapes than s and p orbitals. They include:
- dz²: A dumbbell shape along the z-axis with a torus (doughnut shape) around the center.
- dx²-y²: Two dumbbells along the x and y axes.
- dxy: Four lobes positioned between the x and y axes.
- dxz: Four lobes positioned between the x and z axes.
- dyz: Four lobes positioned between the y and z axes. These orbital sketches often involve four lobes (two positive and two negative regions), emphasizing the regions where the probability of finding the electron is high.
-
Higher n d orbitals: These also exist but are less frequently discussed in introductory chemistry.
f Orbitals (l = 3) and beyond
f orbitals (and orbitals with higher l values) possess even more complex shapes and multiple nodal surfaces. Their sketches are rarely seen in introductory courses due to their intricacy, but they are crucial for understanding the behavior of elements in the f-blocks (lanthanides and actinides).
Selecting Appropriate Sketches: Context is Key
The choice of sketch depends heavily on the context and level of detail required.
-
Introductory Chemistry: Simplified sketches emphasizing the overall shape and orientation of the orbitals are sufficient. The focus is on understanding the general characteristics and relative sizes.
-
Advanced Chemistry and Physics: More detailed sketches might be needed, potentially including nodal surfaces and contour lines representing different probability densities. These sketches aim for a more precise representation of the electron distribution.
-
Qualitative Understanding: For a general understanding of bonding and molecular geometry, simplified sketches are perfectly adequate. The crucial aspect is grasping the relative orientations and overlaps between orbitals.
-
Quantitative Calculations: For computational chemistry and advanced quantum mechanical studies, sketches serve mainly as visual aids to accompany mathematical representations.
Interpreting Orbital Sketches: Common Pitfalls
-
2D vs 3D: Many sketches are 2D representations of 3D shapes. It's essential to visualize the three-dimensional nature of the orbitals. Consider rotating the sketch mentally to understand its full shape.
-
Probability Density: Remember that the sketches represent regions of high electron probability, not the exact location of the electron. The electron can be found anywhere in space, but the probability is highest within the sketched region.
-
Nodal Surfaces: These are areas where the probability of finding the electron is zero. Their presence signifies a change in the sign of the wave function, which is crucial for understanding bonding interactions.
-
Color Coding: Sketches often use color-coding to differentiate between regions of positive and negative wave function values. This is important for understanding how orbitals interact during bond formation.
-
Scale and Relative Size: The relative sizes of orbitals in a sketch might not be to scale. Focus on the relative shapes and orientations rather than the exact dimensions.
Advanced Concepts and Applications
-
Hybrid Orbitals: These are formed by the combination of atomic orbitals, resulting in new orbitals with different shapes and orientations that are more suitable for bonding. Sketches of hybrid orbitals (like sp, sp², sp³, etc.) are crucial for explaining molecular geometries.
-
Molecular Orbitals: These are formed by the combination of atomic orbitals from different atoms. Sketches of molecular orbitals help illustrate the bonding in molecules.
-
Electron Density Maps: These provide a more complete picture of electron distribution in molecules, going beyond simple orbital sketches. They often are produced from computational chemistry.
-
Spectroscopy: Understanding orbital shapes and energies is critical for interpreting spectroscopic data, such as UV-Vis and NMR spectroscopy.
Conclusion
Selecting appropriate sketches of 3D orbitals is a crucial skill for anyone studying chemistry or physics at any level. By understanding the underlying quantum mechanical principles and interpreting sketches correctly, we can gain invaluable insights into the structure and behavior of atoms and molecules. Remember that sketches are simplified representations, and a complete understanding requires a combination of visual and mathematical approaches. As you progress in your studies, you will encounter more sophisticated visualizations and learn to use them effectively to model and understand the complex world of atomic and molecular interactions.
Latest Posts
Latest Posts
-
Introduction To Public Health 6th Edition Pdf
Apr 01, 2025
-
You Must Encrypt Files With Any Of These Extensions
Apr 01, 2025
-
Which Of The Following Pairs Of Terms Is Mismatched
Apr 01, 2025
-
A Government Budget Deficit Exists When
Apr 01, 2025
-
In This Problem A B C And D
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Select The Sketches Of A 3d Orbital. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
