Ring Opening Arrow Pushing Mechanism Under Basic Conditions Haworyh
Holbox
Mar 13, 2025 · 5 min read
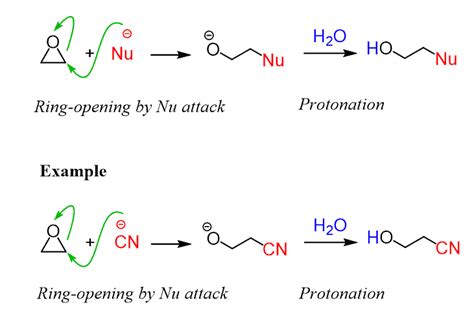
Table of Contents
- Ring Opening Arrow Pushing Mechanism Under Basic Conditions Haworyh
- Table of Contents
- Ring-Opening Reactions of Epoxides Under Basic Conditions: A Comprehensive Guide
- The Mechanism: A Step-by-Step Analysis
- Factors Influencing Regio- and Stereoselectivity
- Synthetic Applications: A Diverse Range of Transformations
- Advanced Topics and Considerations
- Conclusion
- Latest Posts
- Related Post
Ring-Opening Reactions of Epoxides Under Basic Conditions: A Comprehensive Guide
The ring-opening of epoxides under basic conditions represents a fundamental transformation in organic chemistry, offering a versatile route to a wide array of valuable compounds. This reaction, often catalyzed by a nucleophile, proceeds via an SN2-like mechanism, leading to regioselective and stereoselective product formation. Understanding the intricacies of this mechanism is crucial for synthetic chemists aiming to design efficient and selective transformations. This comprehensive guide will delve into the mechanistic details of epoxide ring-opening under basic conditions, exploring the factors influencing regio- and stereoselectivity, and highlighting its synthetic applications.
The Mechanism: A Step-by-Step Analysis
The ring-opening of an epoxide under basic conditions typically involves a two-step process:
Step 1: Nucleophilic Attack
The reaction initiates with the nucleophilic attack of a base (e.g., hydroxide ion, alkoxide ion, thiolate ion) on the less hindered carbon atom of the epoxide ring. This carbon, possessing a partial positive charge due to the electron-withdrawing effect of the oxygen atom, experiences increased electrophilicity. The nucleophile's lone pair attacks this carbon, forming a new carbon-nucleophile bond and simultaneously breaking one of the C-O bonds of the epoxide ring. This step is concerted and follows an SN2-like mechanism, leading to inversion of configuration at the attacked carbon center. The transition state involves a backside attack of the nucleophile, leading to a three-membered ring structure where the nucleophile, carbon, and oxygen are in a linear arrangement.
Step 2: Protonation
The resulting alkoxide intermediate, possessing a negative charge on the oxygen atom, is then protonated by a proton source (often the solvent or a weak acid), yielding the final ring-opened product. This protonation step regenerates the catalytic base and completes the reaction.
Illustrative Example: Ring Opening of Propylene Oxide with Hydroxide Ion
Let's consider the ring-opening of propylene oxide (methyl oxirane) using hydroxide ion as the nucleophile. The hydroxide ion attacks the less hindered primary carbon, leading to inversion of configuration at that carbon. Subsequent protonation yields the 1-propanol product. This example showcases the typical regioselectivity observed in epoxide ring-opening reactions under basic conditions – the nucleophile preferentially attacks the less substituted carbon.
Factors Influencing Regio- and Stereoselectivity
Several factors significantly influence the regio- and stereoselectivity of epoxide ring-opening under basic conditions:
1. Steric Hindrance: The nucleophile preferentially attacks the less hindered carbon atom of the epoxide ring. This preference is largely driven by steric factors; bulky nucleophiles show an even stronger preference for the less hindered site.
2. Electronic Effects: Electron-withdrawing groups (EWGs) on the epoxide ring can influence regioselectivity by directing nucleophilic attack towards the carbon atom bearing the EWG. This is due to the increased electrophilicity of this carbon atom.
3. Nucleophile Strength: Stronger nucleophiles generally react faster and show less sensitivity to steric effects. Therefore, the regioselectivity might be less pronounced with stronger nucleophiles.
4. Solvent Effects: The solvent can play a role in both regio- and stereoselectivity. Protic solvents can influence the solvation of the nucleophile and the transition state, potentially impacting the reaction rate and selectivity.
5. Presence of a Catalyst: The use of catalysts can significantly influence the regioselectivity and reaction rate. For example, certain metal catalysts can coordinate to the epoxide ring, influencing the transition state and promoting regioselective ring opening.
Stereoselectivity: The SN2-like mechanism typically leads to inversion of configuration at the carbon atom undergoing nucleophilic attack. However, if the epoxide is chiral, the stereochemistry of the starting material can also affect the overall stereochemistry of the product. In the case of a chiral epoxide, the ring-opening can lead to diastereomers, with the relative abundance dictated by the factors mentioned above.
Synthetic Applications: A Diverse Range of Transformations
The versatility of epoxide ring-opening under basic conditions makes it a valuable tool in organic synthesis, providing access to a diverse range of functional groups and molecular architectures:
1. Synthesis of Alcohols: As demonstrated earlier, ring-opening with hydroxide or alkoxide ions provides a straightforward route to alcohols. The regioselectivity can be controlled by choosing appropriate reaction conditions and substrates.
2. Synthesis of Ethers: Using alkoxides as nucleophiles enables the synthesis of various ethers. This reaction is particularly useful for the synthesis of symmetrical and unsymmetrical ethers.
3. Synthesis of Thiols: Thiolate ions can be employed as nucleophiles, resulting in the formation of thiols. This pathway offers access to sulfur-containing compounds, vital in numerous applications.
4. Synthesis of Amines: Amines can be synthesized through the use of nitrogen-based nucleophiles such as azide ions or ammonia. This route is a cornerstone in the construction of various nitrogen-containing heterocycles.
Advanced Topics and Considerations
1. Asymmetric Epoxide Ring Opening: The development of asymmetric epoxide ring-opening reactions has been a major area of research, focusing on achieving high enantioselectivity in the formation of chiral alcohols. This area utilizes chiral catalysts and ligands to achieve this selectivity.
2. Regioselective Ring Opening of Unsymmetrical Epoxides: Predicting and controlling the regioselectivity of ring-opening in unsymmetrical epoxides remains a challenge, demanding a thorough understanding of steric and electronic factors.
3. Tandem Reactions: Epoxide ring-opening can be integrated into more complex reaction sequences, enabling the synthesis of intricate molecular structures.
4. Kinetic vs. Thermodynamic Control: Depending on the reaction conditions, either kinetic or thermodynamic control can govern the regioselectivity of the ring-opening process. Understanding these factors is crucial for predicting and controlling product distribution.
Conclusion
The ring-opening of epoxides under basic conditions is a powerful and versatile reaction with broad applications in organic synthesis. A comprehensive understanding of the underlying mechanism, the factors influencing regio- and stereoselectivity, and the diverse range of synthetic applications is essential for any organic chemist. This detailed exploration provides a solid foundation for comprehending and applying this transformative reaction in the design and execution of synthetic strategies. Continued research in this area continues to push the boundaries of selectivity and efficiency, providing ever more powerful tools for organic synthesis. Further investigations into advanced areas, such as the development of new catalysts and the understanding of complex reaction mechanisms, promise even greater advancements in the future. The insights provided in this guide lay the groundwork for a deeper understanding of this fundamental transformation, enabling researchers and students to further explore its vast potential.
Latest Posts
Related Post
Thank you for visiting our website which covers about Ring Opening Arrow Pushing Mechanism Under Basic Conditions Haworyh . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.