Question Volkswagen Draw The Major Sn2
Holbox
Mar 12, 2025 · 5 min read
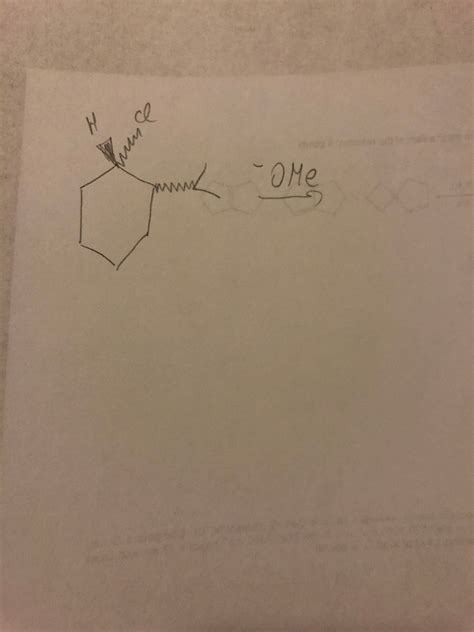
Table of Contents
- Question Volkswagen Draw The Major Sn2
- Table of Contents
- Question: Volkswagen - Draw the Major SN2 Product
- Understanding SN2 Reactions: A Deep Dive
- Key Characteristics of SN2 Reactions:
- Predicting SN2 Products: A Step-by-Step Approach
- Expanding on the "Volkswagen" Question
- Beyond the Basics: Factors Affecting SN2 Reaction Rates
- Conclusion: Applying SN2 Knowledge
- Latest Posts
- Related Post
Question: Volkswagen - Draw the Major SN2 Product
The question, "Volkswagen - Draw the major SN2 product," is a bit of a trick question, cleverly combining a car brand with an organic chemistry concept. It highlights the importance of understanding reaction mechanisms and predicting products in organic chemistry, especially within the context of SN2 reactions. Let's break this down and explore the broader context of SN2 reactions to understand how to approach such a question effectively, even if the prompt lacks a specific reactant.
Understanding SN2 Reactions: A Deep Dive
The SN2 reaction, or bimolecular nucleophilic substitution, is a fundamental concept in organic chemistry. It's a one-step process where a nucleophile attacks an electrophilic carbon atom simultaneously with the departure of a leaving group. This concerted mechanism is crucial in understanding the stereochemistry and regiochemistry of the reaction.
Key Characteristics of SN2 Reactions:
-
Concerted Mechanism: The nucleophilic attack and the departure of the leaving group occur in a single, coordinated step. This means the transition state involves both the nucleophile and the leaving group.
-
Bimolecular: The rate of the reaction depends on the concentration of both the substrate (alkyl halide or tosylate) and the nucleophile. This is reflected in the rate law: Rate = k[substrate][nucleophile].
-
Stereochemistry: SN2 reactions proceed with inversion of configuration at the chiral carbon center. This means if the starting material is chiral, the product will have the opposite stereochemistry. This is a hallmark of the SN2 mechanism.
-
Steric Hindrance: The reaction is sensitive to steric hindrance. Tertiary alkyl halides generally do not undergo SN2 reactions due to the significant steric crowding around the carbon atom. Primary alkyl halides are the most reactive, followed by secondary alkyl halides.
-
Nucleophile Strength: Strong nucleophiles are favored in SN2 reactions. The strength of a nucleophile is often related to its basicity, but there are exceptions. Common nucleophiles include hydroxide ion (OH-), alkoxide ions (RO-), cyanide ion (CN-), azide ion (N3-), and halide ions (I-, Br-, Cl-).
-
Leaving Group Ability: A good leaving group is essential for an SN2 reaction to occur efficiently. Good leaving groups are weak bases, such as halides (I-, Br-, Cl-), tosylates (OTs), and mesylates (OMs).
Predicting SN2 Products: A Step-by-Step Approach
To predict the major SN2 product, you need a specific substrate and nucleophile. Let's consider a hypothetical example to illustrate the process:
Example: Consider the reaction of bromomethane (CH3Br) with sodium hydroxide (NaOH).
-
Identify the Substrate and Nucleophile: In this example, bromomethane (CH3Br) is the substrate, and hydroxide ion (OH-) is the nucleophile. Bromine is a good leaving group.
-
Determine the Nucleophilic Attack: The hydroxide ion (OH-) will attack the carbon atom bonded to the bromine atom. This is because the carbon atom is electrophilic due to the electronegativity difference between carbon and bromine.
-
Predict the Product: The hydroxide ion will replace the bromine atom, leading to the formation of methanol (CH3OH). Remember that the reaction occurs with inversion of configuration. However, since bromomethane is achiral, inversion doesn't affect the product's structure.
-
Consider Steric Hindrance: Since bromomethane is a primary alkyl halide, steric hindrance is minimal, and the SN2 reaction proceeds readily.
Expanding on the "Volkswagen" Question
The initial question, "Volkswagen - Draw the major SN2 product," is intended to provoke thought. Without specifying reactants, it cannot be answered directly. However, it serves as a good exercise in considering various aspects of SN2 reactions.
To make this question answerable, we need to add a reactant. Let's assume the question implies:
"Given a suitable alkyl halide and a strong nucleophile, draw the major SN2 product."
In this case, we can propose scenarios and illustrate the SN2 reaction mechanism.
Scenario 1: Primary Alkyl Halide
Let's use 1-chlorobutane (CH3CH2CH2CH2Cl) reacting with sodium iodide (NaI) in acetone.
-
Substrate and Nucleophile: 1-chlorobutane is the substrate, and iodide (I-) is the nucleophile.
-
Nucleophilic Attack: The iodide ion attacks the carbon atom bonded to the chlorine atom.
-
Product: 1-iodobutane (CH3CH2CH2CH2I) is formed with inversion of configuration. Since the carbon is not chiral, there is no change in stereoisomerism.
Scenario 2: Secondary Alkyl Halide
Let's use 2-bromobutane (CH3CHBrCH2CH3) reacting with potassium cyanide (KCN) in DMSO.
-
Substrate and Nucleophile: 2-bromobutane is the substrate, and cyanide (CN-) is the nucleophile.
-
Nucleophilic Attack: The cyanide ion attacks the chiral carbon atom bonded to the bromine atom.
-
Product: The major product is 2-methylbutanenitrile (CH3CH(CN)CH2CH3) with inversion of configuration at the chiral carbon. The original stereochemistry is inverted in the product. If the reactant was (R)-2-bromobutane, the product would be (S)-2-methylbutanenitrile.
Scenario 3: The Challenge of Tertiary Alkyl Halides
Let's attempt to use 2-chloro-2-methylpropane (t-butyl chloride) with sodium methoxide (NaOCH3). This will not yield an SN2 product. The steric hindrance around the tertiary carbon prevents the nucleophile from approaching closely enough to initiate the concerted mechanism. Instead, an E2 elimination reaction is more likely to occur.
This demonstrates that the feasibility of SN2 depends on the structure of the alkyl halide.
Beyond the Basics: Factors Affecting SN2 Reaction Rates
Several factors influence the rate of an SN2 reaction:
-
Solvent Effects: Polar aprotic solvents, such as DMSO, DMF, and acetone, are preferred for SN2 reactions because they solvate the cation (e.g., Na+) more effectively than the nucleophile, increasing the nucleophile's reactivity. Polar protic solvents, like water and alcohols, can hinder the SN2 reaction by solvating the nucleophile.
-
Leaving Group Ability: The better the leaving group, the faster the reaction. This is because a good leaving group stabilizes the negative charge in the transition state. The order of leaving group ability is typically: I- > Br- > Cl- > F-.
-
Nucleophile Strength: Stronger nucleophiles react faster. Nucleophilicity is related to basicity, but it is not directly proportional. Factors like steric hindrance and polarizability play a role.
Conclusion: Applying SN2 Knowledge
The "Volkswagen" question serves as a useful reminder of the core principles behind SN2 reactions. Understanding the mechanism, the effects of steric hindrance, nucleophilicity, leaving group ability, and solvent effects is vital for predicting the products of these reactions. By systematically analyzing the substrate and nucleophile, and considering these factors, one can accurately predict the major SN2 product, and by extension, understand the broader applications of this critical reaction in organic chemistry and beyond. Always remember to explicitly define your reactants to get a definitive answer.
Latest Posts
Related Post
Thank you for visiting our website which covers about Question Volkswagen Draw The Major Sn2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.