Question Chevy You Are Given A Nucleophile And A Substrate
Holbox
Mar 12, 2025 · 6 min read
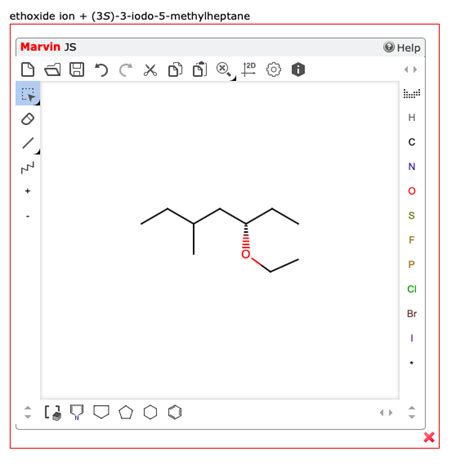
Table of Contents
Question: You are given a nucleophile and a substrate. Predict the outcome of the reaction.
This question forms the cornerstone of a vast area of organic chemistry: nucleophilic substitution and addition reactions. Understanding how nucleophiles interact with substrates is crucial for predicting reaction products and designing synthetic routes. This in-depth exploration will delve into the factors that govern these reactions, focusing on the key elements of substrate structure, nucleophile strength, leaving group ability, and reaction conditions. We'll explore different reaction mechanisms and provide examples to illustrate the concepts.
Understanding Nucleophiles and Substrates
Before predicting reaction outcomes, we need to define our terms:
Nucleophiles: Electron-Rich Species
A nucleophile (literally, "nucleus-loving") is a chemical species that donates an electron pair to an electrophile to form a chemical bond. Nucleophiles are characterized by their electron density; they possess lone pairs or readily available pi electrons. The strength of a nucleophile is influenced by several factors:
- Charge: Negatively charged nucleophiles are generally stronger than neutral nucleophiles. For example, hydroxide ion (OH⁻) is a stronger nucleophile than water (H₂O).
- Electronegativity: Less electronegative atoms are better nucleophiles. This is because they hold their electrons less tightly and are more readily available for donation. For example, iodide (I⁻) is a better nucleophile than fluoride (F⁻).
- Steric hindrance: Bulky nucleophiles can be less reactive due to steric crowding around the nucleophilic center. For example, tert-butoxide (t-BuO⁻) is a weaker nucleophile than methoxide (MeO⁻) due to the bulky tert-butyl group.
- Solvent effects: The solvent can significantly impact nucleophile strength. Polar protic solvents (like water or alcohols) can solvate nucleophiles, reducing their reactivity. Polar aprotic solvents (like DMSO or DMF) tend to enhance nucleophilicity by reducing solvation.
Examples of Nucleophiles: OH⁻, CN⁻, I⁻, Br⁻, Cl⁻, SH⁻, RS⁻, NH₃, RNH₂, H₂O, ROH.
Substrates: Electron-Deficient Species
The substrate is the molecule that undergoes a nucleophilic attack. Typically, substrates possess an electrophilic center, often a carbon atom bonded to a good leaving group. The nature of the substrate significantly dictates the reaction mechanism and outcome. Key aspects include:
- Leaving group ability: The leaving group is an atom or group that departs from the substrate, taking the electron pair with it. Good leaving groups are generally weak bases, meaning they are stable when they depart with a negative charge. Examples of good leaving groups include halides (I⁻, Br⁻, Cl⁻), tosylate (OTs), and mesylate (OMs). Poor leaving groups are often strong bases and are less likely to participate in nucleophilic substitution reactions.
- Substrate structure: The carbon atom undergoing the nucleophilic attack can be primary (methyl or one other alkyl substituent), secondary (two other alkyl substituents), or tertiary (three other alkyl substituents). This dramatically influences the reaction mechanism and stereochemistry.
- Steric hindrance: Bulky substituents around the electrophilic center can hinder nucleophilic attack, slowing down the reaction rate.
Nucleophilic Substitution Reactions: SN1 and SN2
Nucleophilic substitution reactions involve the replacement of a leaving group by a nucleophile. Two primary mechanisms govern these reactions: SN1 and SN2.
SN2 Reactions: Concerted Mechanism
SN2 (substitution nucleophilic bimolecular) reactions are concerted, meaning that the nucleophile attacks the substrate simultaneously as the leaving group departs. This results in a single, transition state.
- Stereochemistry: SN2 reactions proceed with inversion of configuration. If the substrate is chiral, the product will have the opposite stereochemistry.
- Kinetics: SN2 reactions are second-order, meaning the rate depends on the concentration of both the nucleophile and the substrate: Rate = k[nucleophile][substrate].
- Substrate preference: SN2 reactions favor primary substrates and are less favored for secondary and tertiary substrates due to steric hindrance.
- Nucleophile strength: SN2 reactions are favored by strong nucleophiles.
SN1 Reactions: Two-Step Mechanism
SN1 (substitution nucleophilic unimolecular) reactions proceed through a two-step mechanism:
- Ionization: The leaving group departs, forming a carbocation intermediate.
- Nucleophilic attack: The nucleophile attacks the carbocation.
- Stereochemistry: SN1 reactions result in racemization, producing a mixture of stereoisomers. This is because the planar carbocation intermediate can be attacked from either side.
- Kinetics: SN1 reactions are first-order, meaning the rate depends only on the concentration of the substrate: Rate = k[substrate].
- Substrate preference: SN1 reactions favor tertiary substrates, which form relatively stable carbocations. Primary substrates rarely undergo SN1 reactions.
- Nucleophile strength: SN1 reactions are less sensitive to nucleophile strength than SN2 reactions. Weak nucleophiles can participate in SN1 reactions.
Predicting Reaction Outcomes: A Step-by-Step Approach
To predict the outcome of a nucleophilic substitution reaction, consider the following factors:
- Identify the nucleophile and substrate: Determine the strength of the nucleophile and the nature of the substrate (primary, secondary, tertiary; leaving group ability; steric hindrance).
- Determine the likely mechanism: Based on the substrate structure and nucleophile strength, predict whether the reaction will proceed via SN1 or SN2. Strong nucleophiles and primary substrates favor SN2; weak nucleophiles and tertiary substrates favor SN1. Secondary substrates can undergo either SN1 or SN2 depending on the specific conditions (nucleophile strength, solvent).
- Predict the product: Based on the chosen mechanism, predict the structure and stereochemistry of the product. Remember SN2 reactions result in inversion of configuration, while SN1 reactions lead to racemization.
- Consider solvent effects: Polar protic solvents favor SN1 reactions, while polar aprotic solvents favor SN2 reactions.
Examples
Example 1: Reaction of bromomethane (CH₃Br) with sodium iodide (NaI) in acetone.
- Nucleophile: I⁻ (strong nucleophile)
- Substrate: CH₃Br (primary substrate, good leaving group)
- Mechanism: SN2
- Product: CH₃I (iodomethane) with inversion of configuration (although bromomethane is achiral, the principle applies if the substrate were chiral).
Example 2: Reaction of tert-butyl bromide ((CH₃)₃CBr) with methanol (CH₃OH) in water.
- Nucleophile: CH₃OH (weak nucleophile)
- Substrate: (CH₃)₃CBr (tertiary substrate, good leaving group)
- Mechanism: SN1
- Product: (CH₃)₃COCH₃ (tert-butyl methyl ether) and (CH₃)₃COH (tert-butyl alcohol), a racemic mixture due to the planar carbocation intermediate.
Example 3: Reaction of 2-bromobutane with potassium cyanide (KCN) in DMSO.
- Nucleophile: CN⁻ (strong nucleophile)
- Substrate: 2-bromobutane (secondary substrate, good leaving group)
- Mechanism: Likely SN2, but some SN1 character is possible depending on the reaction conditions. The strong nucleophile and aprotic solvent favor SN2.
- Product: A mixture of 2-cyanobutane, with a predominance of the inverted stereoisomer. A small amount of racemization might occur due to potential SN1 character.
Nucleophilic Addition Reactions
Nucleophilic addition reactions are another important class of reactions involving nucleophiles. These reactions typically occur with carbonyl compounds (aldehydes and ketones) and other electron-deficient pi systems.
The nucleophile attacks the electrophilic carbon atom of the carbonyl group, forming a tetrahedral intermediate. This intermediate can then undergo various transformations, depending on the specific reaction conditions.
Conclusion
Predicting the outcome of a reaction involving a nucleophile and a substrate requires a comprehensive understanding of several factors including the nucleophile's strength, the substrate's structure, the leaving group's ability, and reaction conditions. By carefully considering these factors, we can effectively predict the mechanism (SN1 or SN2 or nucleophilic addition), product structure, and stereochemistry of the reaction. This knowledge is fundamental to organic synthesis and allows for the rational design of synthetic routes towards target molecules. Remember to always consider the interplay between these factors for a precise prediction. Further exploration into specific reaction types and detailed mechanistic studies will provide a deeper understanding of this fascinating field of chemistry.
Latest Posts
Latest Posts
-
Eocs Receive Senior Level Guidance From
Mar 12, 2025
-
A User Receives This Error Message Not Secure
Mar 12, 2025
-
How To Connect Chegg To Tinder
Mar 12, 2025
-
How To Delete Question From Chegg
Mar 12, 2025
-
How To Delete A Chegg Question
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Question Chevy You Are Given A Nucleophile And A Substrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
