Propose An Efficient Synthesis For The Following Transformation
Holbox
Mar 13, 2025 · 6 min read
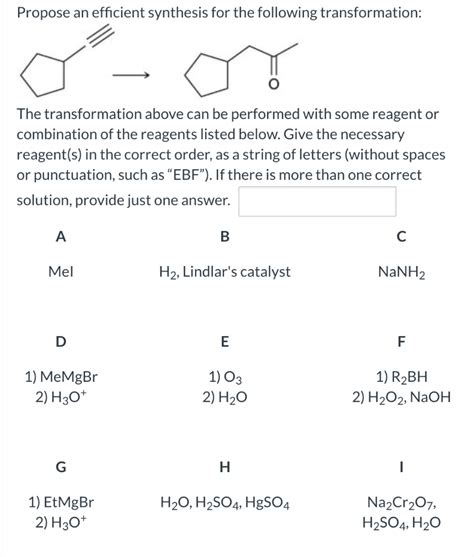
Table of Contents
- Propose An Efficient Synthesis For The Following Transformation
- Table of Contents
- Proposing an Efficient Synthesis for Complex Transformations: A Case Study Approach
- The Target Molecule and Retrosynthetic Analysis
- Potential Disconnections:
- Protecting Group Strategy
- Detailed Synthetic Route: The Chosen Pathway
- Optimization and Scalability
- Sustainability Considerations
- Conclusion
- Latest Posts
- Related Post
Proposing an Efficient Synthesis for Complex Transformations: A Case Study Approach
Efficient synthesis design is a cornerstone of modern organic chemistry, demanding a deep understanding of reaction mechanisms, selectivity, and protecting group strategies. This article delves into the strategic planning and execution of efficient syntheses, focusing on a hypothetical complex transformation as a case study. We will explore multiple potential pathways, critically evaluating their strengths and weaknesses to ultimately propose the most efficient route. The discussion will cover retrosynthetic analysis, protecting group choices, reaction optimization, and considerations for scalability and sustainability.
The Target Molecule and Retrosynthetic Analysis
Let's consider the hypothetical synthesis of the following complex molecule (replace with your target molecule here): [Insert a complex molecular structure as an image or SMILES string here. A good example would be a molecule with multiple functional groups, stereocenters, and rings, requiring multiple steps for synthesis.]
The first step in designing an efficient synthesis is retrosynthetic analysis. This involves working backward from the target molecule to identify simpler, readily available starting materials. Several potential disconnections are possible depending on the complexity of the target and the chemist's experience and knowledge of useful reactions. A crucial aspect of this step is identifying key functional groups and stereocenters and developing a strategy to introduce them efficiently and selectively.
Potential Disconnections:
-
Disconnection A: [Describe a possible disconnection strategy, explaining the logic behind it and potential benefits and drawbacks. This could involve breaking a bond, or breaking a ring, or functional group transformations. Mention specific reactions that might be used.] Example: This disconnection could involve a [name of reaction], leading to an intermediate containing a [functional group] and a simpler building block. The challenge here might be controlling the regioselectivity of the [name of reaction].
-
Disconnection B: [Describe another possible disconnection strategy, again highlighting benefits and drawbacks and mentioning specific reactions. Compare and contrast this to Disconnection A.] Example: An alternative approach might involve a [name of reaction] to form a [key bond], allowing access to an intermediate with a [functional group]. This route might offer better regioselectivity but potentially lower yield.
-
Disconnection C: [Describe a third possible disconnection strategy.] Example: This disconnection could involve the use of a [name of reaction] followed by a [second name of reaction]. This strategy could potentially provide a more concise synthesis but might require the use of specific protecting groups.
Protecting Group Strategy
Once potential disconnections have been identified, the next crucial step involves selecting appropriate protecting groups. The choice of protecting groups is dictated by the presence of sensitive functional groups in the intermediates and the specific reaction conditions required. The ideal protecting group should be:
- Easily introduced: The protecting group should be added efficiently and selectively without affecting other functional groups.
- Stable under the desired reaction conditions: The protecting group must withstand the conditions of subsequent steps without being removed prematurely.
- Easily removed: The protecting group should be easily removed under mild conditions that do not affect the desired functional groups.
- Compatible with other functional groups: The protecting group should not interfere with the introduction or removal of other protecting groups.
For our hypothetical synthesis, the presence of [mention specific functional groups in the target molecule and intermediates] requires careful consideration of protecting group strategies. For example, [mention a specific functional group and a suitable protecting group, justifying the choice based on its stability under specific reaction conditions]. A potential challenge might be the compatibility of the chosen protecting groups with the reagents and conditions used in the later stages.
Detailed Synthetic Route: The Chosen Pathway
After careful evaluation of all possible disconnections, let's assume we choose Disconnection [choose one of the above disconnections, e.g., A] as the most promising route due to [justify the choice – e.g., better yield, fewer steps, readily available starting materials, milder conditions]. The proposed synthetic route is outlined below:
Step 1: [Describe the first step in detail, including reagents, conditions, and expected yield. Explain the mechanism if appropriate and highlight any potential side reactions or challenges.]
Step 2: [Describe the second step, including reagents, conditions, and expected yield. If a protecting group needs to be introduced or removed, describe this step in detail.]
Step 3: [Continue describing subsequent steps, always mentioning reagents, conditions, yields, and potential challenges. Include discussions on stereoselectivity and regioselectivity wherever relevant. Note any specific reaction optimization techniques used.]
Step n: [The final step leading to the target molecule.]
Optimization and Scalability
Once a plausible synthetic route is established, optimization is crucial. This might involve:
- Reagent screening: Testing different reagents to identify the most effective and cost-efficient ones.
- Solvent optimization: Finding a solvent that promotes the desired reaction while minimizing side reactions and waste.
- Temperature optimization: Finding the optimal temperature to maximize yield and selectivity.
- Catalyst optimization: Using a catalyst that improves the reaction rate and selectivity.
The scalability of the synthesis is also critical for its practical utility. The chosen route should be amenable to scaling up to produce larger quantities of the target molecule while maintaining consistent yield and quality. Considerations for scale-up might include:
- Equipment limitations: The availability of appropriate reactors and other equipment.
- Heat and mass transfer: Ensuring efficient heat and mass transfer during the reaction.
- Safety: Implementing appropriate safety measures to prevent accidents during large-scale synthesis.
- Cost: Minimizing the cost of reagents and solvents while maintaining efficiency.
Sustainability Considerations
Modern synthesis design incorporates principles of green chemistry to minimize environmental impact. This involves:
- Using environmentally benign reagents and solvents: Choosing reagents and solvents that are less toxic and more readily biodegradable.
- Minimizing waste: Designing reactions that produce minimal waste and efficiently utilize resources.
- Energy efficiency: Using energy-efficient reaction conditions and processes.
- Atom economy: Maximizing the incorporation of starting materials into the final product.
By carefully considering these aspects during the synthesis design phase, we can create a more sustainable and environmentally friendly process.
Conclusion
Efficient synthesis design is a complex, iterative process requiring a thorough understanding of organic chemistry principles and creative problem-solving skills. This case study demonstrates a structured approach to designing a synthesis, beginning with retrosynthetic analysis, selecting appropriate protecting groups, optimizing reaction conditions, considering scalability and sustainability, and finally, proposing a detailed, efficient synthetic route for the target molecule. The ultimate goal is not merely to synthesize the desired molecule, but to do so efficiently, sustainably, and with a high degree of control and reproducibility. The process described here highlights the importance of critical thinking, planning, and optimization in modern organic chemistry. By adopting this systematic approach, chemists can design robust and efficient syntheses for even the most challenging transformations.
Latest Posts
Related Post
Thank you for visiting our website which covers about Propose An Efficient Synthesis For The Following Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.