Predict The Product For The Reaction Shown.
Holbox
Mar 17, 2025 · 5 min read
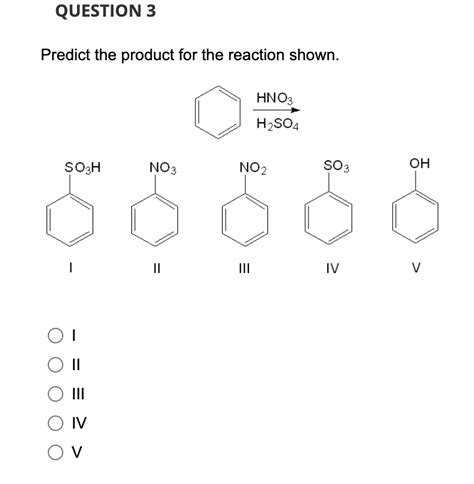
Table of Contents
Predicting the Product for a Given Reaction: A Comprehensive Guide
Predicting the product of a chemical reaction is a fundamental skill in chemistry. It requires a thorough understanding of reaction mechanisms, functional groups, and reaction conditions. While memorizing individual reactions is helpful, a deeper understanding of underlying principles allows for accurate prediction even for unfamiliar reactions. This guide explores various strategies and approaches for accurately predicting reaction products, covering a wide range of reaction types.
Understanding Reaction Mechanisms: The Key to Prediction
Before delving into specific reactions, let's establish the importance of understanding reaction mechanisms. A reaction mechanism describes the step-by-step process by which reactants transform into products. Knowing the mechanism unveils the intermediate species formed and the sequence of bond-breaking and bond-forming events. This knowledge is crucial for accurate product prediction.
Common Reaction Mechanisms:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism involves a two-step process: the departure of a leaving group to form a carbocation intermediate, followed by nucleophilic attack. The rate-determining step is the formation of the carbocation. Therefore, stability of the carbocation (tertiary > secondary > primary) dictates the reaction outcome. Rearrangements are often observed in SN1 reactions.
-
SN2 (Substitution Nucleophilic Bimolecular): This mechanism is a concerted one-step process where the nucleophile attacks the substrate simultaneously as the leaving group departs. The reaction rate depends on both the nucleophile and substrate concentrations. SN2 reactions typically favor primary substrates and strong nucleophiles. Stereochemistry is inverted in SN2 reactions.
-
E1 (Elimination Unimolecular): Similar to SN1, E1 reactions proceed through a carbocation intermediate. However, instead of nucleophilic attack, a base abstracts a proton, leading to the formation of a double bond. The stability of the carbocation influences the location of the double bond (Zaitsev's rule).
-
E2 (Elimination Bimolecular): This is a concerted one-step process where the base abstracts a proton and the leaving group departs simultaneously. The stereochemistry of the reactants plays a critical role in determining the product. Anti-periplanar geometry is preferred for E2 reactions.
-
Addition Reactions: These reactions involve the addition of atoms or groups to a multiple bond (e.g., C=C, C≡C, C=O). The type of addition (electrophilic, nucleophilic) depends on the nature of the multiple bond and the reagents. Markovnikov's rule often applies to electrophilic additions to alkenes.
-
Condensation Reactions: These reactions involve the joining of two molecules with the elimination of a small molecule, such as water or alcohol. Examples include esterification, aldol condensation, and Claisen condensation.
Predicting Products: A Step-by-Step Approach
Predicting the product of a reaction involves a systematic approach:
-
Identify the functional groups: Recognize the functional groups present in the reactants. This is the first step in determining the likely reaction type.
-
Determine the reaction type: Based on the functional groups and the reagents used, classify the reaction as SN1, SN2, E1, E2, addition, condensation, or another type. Consider the reaction conditions (solvent, temperature, presence of a catalyst).
-
Predict the mechanism: Understanding the mechanism is crucial for accurate prediction. Draw out the mechanism step-by-step, paying attention to the intermediate species formed.
-
Consider stereochemistry: For reactions involving chiral centers, predict the stereochemistry of the product. SN2 reactions invert stereochemistry, while SN1 reactions can lead to racemization.
-
Apply relevant rules: Utilize rules such as Markovnikov's rule, Zaitsev's rule, and other guiding principles specific to each reaction type.
-
Consider side reactions: Be aware that multiple products might form due to competing reactions or side reactions. Consider the relative rates and yields of different products.
Examples of Product Prediction
Let's illustrate the process with several examples:
Example 1: SN2 Reaction
Reactants: Bromomethane (CH3Br) and sodium hydroxide (NaOH) in ethanol.
Reaction Type: SN2 (strong nucleophile, primary substrate)
Mechanism: The hydroxide ion attacks the carbon atom bonded to the bromine, simultaneously with the departure of the bromide ion.
Product: Methanol (CH3OH) and sodium bromide (NaBr). The stereochemistry is inverted if the starting material was chiral.
Example 2: E1 Reaction
Reactants: 2-bromo-2-methylpropane (tert-butyl bromide) and ethanol.
Reaction Type: E1 (weak nucleophile, tertiary substrate)
Mechanism: The bromide ion leaves, forming a tertiary carbocation. A base (ethanol) abstracts a proton, forming a double bond.
Product: 2-methylpropene (isobutylene) and HBr.
Example 3: Electrophilic Addition to an Alkene
Reactants: Propene (CH3CH=CH2) and hydrogen bromide (HBr).
Reaction Type: Electrophilic addition.
Mechanism: The electrophile (H+) attacks the alkene, forming a carbocation intermediate. The bromide ion then attacks the carbocation.
Product: 2-bromopropane. Markovnikov's rule predicts that the bromine atom adds to the more substituted carbon atom.
Example 4: Aldol Condensation
Reactants: Ethanal (acetaldehyde) in the presence of a base.
Reaction Type: Aldol condensation.
Mechanism: The base deprotonates one molecule of ethanal, forming an enolate ion. The enolate ion attacks the carbonyl group of another ethanal molecule. After dehydration, a α,β-unsaturated aldehyde forms.
Product: 3-hydroxybutanal (initially), followed by dehydration to yield but-2-enal (crotonaldehyde).
Advanced Considerations in Product Prediction
-
Regioselectivity: This refers to the preferential formation of one isomer over another. Markovnikov's rule is an example of regioselectivity in electrophilic addition.
-
Stereoselectivity: This refers to the preferential formation of one stereoisomer over another. SN2 reactions are stereospecific (inversion of configuration), while SN1 reactions often lead to racemization.
-
Chemoselectivity: This is the selective reaction of one functional group in the presence of other functional groups. Protecting groups are often used to achieve chemoselectivity.
-
Kinetic vs. Thermodynamic Control: Some reactions can yield different products depending on the reaction conditions (temperature, time). Kinetic control favors the faster-forming product, while thermodynamic control favors the more stable product.
-
Catalyst Effects: Catalysts can significantly alter the reaction pathway and product distribution.
Conclusion
Predicting the product of a chemical reaction requires a solid understanding of reaction mechanisms, functional groups, and reaction conditions. By following a systematic approach, considering relevant rules and mechanisms, and accounting for potential side reactions, one can accurately predict the products of a vast array of chemical reactions. Remember that practice is key; working through numerous examples is the best way to hone your skills in this vital aspect of organic chemistry. Furthermore, always refer to reliable chemistry textbooks and resources for further clarification and detailed explanations of specific reactions and mechanisms.
Latest Posts
Latest Posts
-
What Is The Correct Label For A
Mar 17, 2025
-
Owner Distribution Is What Kind Of Account
Mar 17, 2025
-
Tom Is Working On A Report That Contains
Mar 17, 2025
-
In Every Choice You Weigh The
Mar 17, 2025
-
While Develping A Segmentation Approvah The Brand
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Predict The Product For The Reaction Shown. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
