Predict The Major Product Of The Following Reactions.
Holbox
Mar 10, 2025 · 6 min read
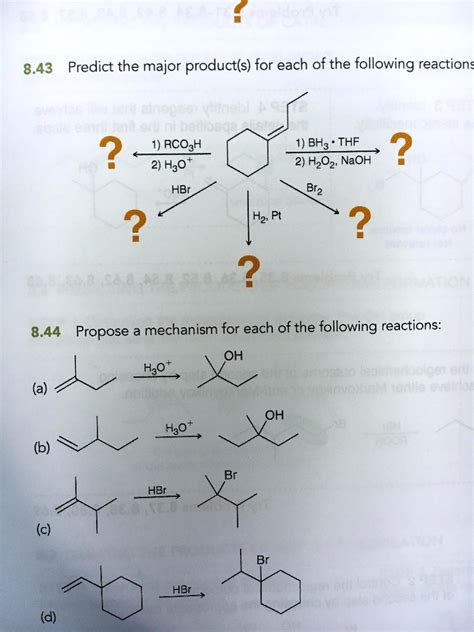
Table of Contents
Predicting the Major Product in Organic Reactions: A Comprehensive Guide
Predicting the major product of a chemical reaction is a cornerstone of organic chemistry. It requires a deep understanding of reaction mechanisms, functional group transformations, and the interplay of various factors influencing reaction pathways. This comprehensive guide will delve into several common reaction types, exploring the principles that govern product formation and offering strategies for accurate prediction. We will consider factors like regioselectivity, stereoselectivity, and chemoselectivity, illustrating with numerous examples.
1. Understanding Reaction Mechanisms: The Foundation of Prediction
Before attempting to predict the major product, a thorough grasp of the reaction mechanism is crucial. The mechanism outlines the step-by-step process of bond breaking and bond formation, revealing the intermediate species involved and their relative stability. This understanding allows us to anticipate the most likely pathway and, consequently, the predominant product.
Example: Consider the SN1 reaction. This mechanism proceeds through a carbocation intermediate. The stability of this carbocation dictates the regioselectivity of the reaction. More substituted carbocations are more stable (due to hyperconjugation and inductive effects), leading to the formation of the most substituted product as the major product.
2. Regioselectivity: Where the Reaction Occurs
Regioselectivity refers to the preferential formation of one regioisomer over another. This is frequently observed in addition reactions to alkenes and alkynes, and in substitution reactions. Understanding the driving forces behind regioselectivity is key to predicting the major product.
Examples:
-
Markovnikov's Rule: In the addition of HX (where X is a halogen) to an alkene, the hydrogen atom adds to the carbon atom that already has more hydrogen atoms, while the halogen adds to the carbon with fewer hydrogens. This is because the more substituted carbocation intermediate is more stable.
-
Anti-Markovnikov Addition: In the presence of peroxides, the addition of HBr to an alkene follows the anti-Markovnikov rule, where the hydrogen atom adds to the carbon with fewer hydrogen atoms. This is due to a radical mechanism involving a more stable tertiary radical intermediate.
-
Electrophilic Aromatic Substitution: In electrophilic aromatic substitution reactions, the electrophile attacks the aromatic ring at the position that leads to the most stable intermediate carbocation (often determined by the directing effects of substituents already present on the ring). Ortho/para directing groups (like -OH, -NH2, -OCH3) favor substitution at ortho and para positions, while meta directing groups (like -NO2, -COOH, -SO3H) favor substitution at the meta position.
3. Stereoselectivity: The Spatial Arrangement of Products
Stereoselectivity refers to the preferential formation of one stereoisomer over another. This is particularly important in reactions that create chiral centers. Understanding the stereochemical outcome requires considering the reaction mechanism and the potential for steric hindrance.
Examples:
-
SN1 Reactions: SN1 reactions generally lead to racemic mixtures because the carbocation intermediate is planar and can be attacked from either side with equal probability.
-
SN2 Reactions: SN2 reactions proceed through a backside attack, leading to inversion of configuration at the chiral center.
-
Addition Reactions to Alkenes: Syn addition results in the addition of two groups to the same face of the alkene, while anti addition results in addition to opposite faces. The stereochemistry depends on the reaction mechanism (e.g., syn addition in hydroboration-oxidation, anti addition in halohydrin formation).
4. Chemoselectivity: Selective Reaction of One Functional Group over Another
Chemoselectivity refers to the preferential reaction of one functional group in the presence of other reactive functional groups. This selectivity is often exploited in organic synthesis to achieve specific transformations without affecting other parts of the molecule.
Examples:
-
Selective Reduction: Different reducing agents exhibit different chemoselectivities. For instance, LiAlH4 reduces esters, ketones, and aldehydes, while NaBH4 selectively reduces aldehydes and ketones but not esters.
-
Selective Protection: Protecting groups are used to temporarily mask reactive functional groups while allowing other reactions to occur. This is crucial for complex syntheses involving multiple functional groups.
5. Predicting Products: A Step-by-Step Approach
To effectively predict the major product of a reaction, follow these steps:
- Identify the functional groups: Determine the reactive functional groups present in the starting material.
- Identify the reagents and conditions: Note the reagents used and the reaction conditions (temperature, solvent, etc.), as these greatly influence the outcome.
- Propose a mechanism: Draw out the mechanism, considering the likely intermediates and transition states. Pay close attention to carbocation stability, radical stability, and steric effects.
- Determine regioselectivity and stereoselectivity: Based on the mechanism, predict where the reaction will occur (regioselectivity) and the resulting stereochemistry (stereoselectivity).
- Consider chemoselectivity: If multiple functional groups are present, determine which will react preferentially under the given conditions.
- Draw the major product: Based on the above considerations, draw the structure of the major product.
6. Advanced Considerations: Equilibrium, Kinetic vs. Thermodynamic Control
Several additional factors can influence the major product formed:
- Equilibrium Control: In reactions that are reversible, the major product is the most thermodynamically stable product.
- Kinetic Control: In reactions that are irreversible or fast, the major product is the one formed fastest (kinetic product). This often means the product formed through the lowest energy transition state.
- Steric Effects: Bulky groups can hinder reactions and influence the regio- and stereoselectivity.
- Solvent Effects: The solvent can significantly influence the reaction rate and selectivity. Polar protic solvents favor SN1 reactions, while polar aprotic solvents favor SN2 reactions.
7. Illustrative Examples
Let's examine a few examples to solidify the concepts discussed:
Example 1: Addition of HBr to propene
The addition of HBr to propene follows Markovnikov's rule. The hydrogen atom adds to the less substituted carbon, forming a more stable secondary carbocation intermediate, resulting in 2-bromopropane as the major product.
Example 2: SN2 Reaction of 2-bromobutane with sodium methoxide
The SN2 reaction of 2-bromobutane with sodium methoxide results in inversion of configuration at the chiral center, leading to the formation of (S)-2-methoxybutane if the starting material was (R)-2-bromobutane.
Example 3: Friedel-Crafts Alkylation of benzene with 1-chloropropane
The Friedel-Crafts alkylation of benzene with 1-chloropropane will lead to the formation of isopropylbenzene (cumene) as the major product due to the formation of a more stable secondary carbocation intermediate.
8. Conclusion
Predicting the major product in organic reactions is a challenging but rewarding skill. By understanding reaction mechanisms, regioselectivity, stereoselectivity, chemoselectivity, and other influencing factors, you can significantly improve your ability to predict the outcome of various organic reactions. Remember to always consider the interplay of these factors and apply a systematic approach to arrive at the most likely major product. Continued practice and problem-solving are crucial for mastering this fundamental aspect of organic chemistry. The more examples you work through, the better your intuition will become in discerning the most likely reaction pathway and, subsequently, the major product. Remember to always check your work and consider alternative pathways, especially in complex reactions.
Latest Posts
Latest Posts
-
List Five Principles For Using Medication To Manage Symptoms
Mar 11, 2025
-
Psychiatric And Mental Health Nursing For Canadian Practice
Mar 11, 2025
-
Plugging Is The Preferred Method Of
Mar 11, 2025
-
When Should You Start Assessing Customers Using The Traffic Light
Mar 11, 2025
-
Formula For Loosest And Densest State Porosity
Mar 11, 2025
Related Post
Thank you for visiting our website which covers about Predict The Major Product Of The Following Reactions. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
