Predict The Major Product Of The Following Reaction.
Holbox
Mar 10, 2025 · 5 min read
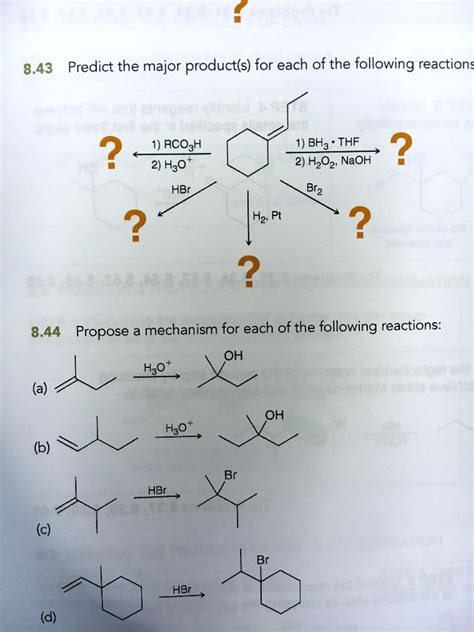
Table of Contents
Predicting the Major Product of Organic Reactions: A Comprehensive Guide
Predicting the major product of an organic reaction is a cornerstone of organic chemistry. It requires a deep understanding of reaction mechanisms, reaction kinetics, thermodynamics, and the interplay of various factors influencing reaction pathways. This article delves into the strategies and considerations needed to accurately predict the major product in various organic reactions. We'll explore several examples, highlighting the reasoning behind our predictions and emphasizing the importance of considering steric hindrance, electronic effects, and reaction conditions.
Understanding Reaction Mechanisms: The Foundation of Prediction
Before attempting to predict the major product, understanding the underlying reaction mechanism is paramount. The mechanism dictates the step-by-step process of bond breaking and formation, leading to the final product(s). Common mechanisms include:
-
SN1 (Substitution Nucleophilic Unimolecular): This two-step mechanism involves a carbocation intermediate. The rate-determining step is the unimolecular ionization of the substrate, making it sensitive to carbocation stability. More stable carbocations (tertiary > secondary > primary) are formed more readily. Rearrangements are often observed.
-
SN2 (Substitution Nucleophilic Bimolecular): This concerted mechanism involves a single transition state where the nucleophile attacks from the backside of the leaving group, leading to inversion of configuration. Steric hindrance significantly affects the rate; bulky substrates react slower.
-
E1 (Elimination Unimolecular): Similar to SN1, this two-step mechanism involves a carbocation intermediate. The rate-determining step is the unimolecular ionization of the substrate. The stability of the carbocation dictates the regioselectivity (Zaitsev's rule favors the more substituted alkene).
-
E2 (Elimination Bimolecular): This concerted mechanism involves a single transition state where the base abstracts a proton and the leaving group departs simultaneously. Stereochemistry is crucial; anti-periplanar geometry is preferred for maximum orbital overlap. Regioselectivity again follows Zaitsev's rule.
-
Addition Reactions: These reactions involve the addition of a reagent across a multiple bond (e.g., alkene, alkyne). Markovnikov's rule often governs regioselectivity in electrophilic additions to alkenes, predicting that the hydrogen atom adds to the carbon atom with the greater number of hydrogen atoms already attached. Anti-Markovnikov addition can occur under radical conditions.
Factors Influencing Product Distribution: Sterics, Electronics, and Kinetics
Several factors beyond the basic mechanism influence the major product formation:
1. Steric Hindrance: The Bulky Factor
Bulky groups hinder the approach of reactants, slowing down reactions and sometimes favoring alternative pathways. In SN2 reactions, bulky substrates react significantly slower than less hindered ones. In elimination reactions, the base's approach can be sterically hindered, leading to a preference for less substituted alkenes under certain conditions.
2. Electronic Effects: Guiding the Reaction Path
Electronic effects influence the stability of intermediates and transition states. Electron-donating groups stabilize carbocations, while electron-withdrawing groups destabilize them. This affects the regioselectivity and stereoselectivity of reactions. For example, in electrophilic aromatic substitution, electron-donating groups direct the electrophile to the ortho and para positions, while electron-withdrawing groups direct it to the meta position.
3. Reaction Conditions: Temperature, Solvent, and Reagents
Reaction conditions play a crucial role. Higher temperatures generally favor faster reactions and often lead to thermodynamically controlled products (more stable products). Lower temperatures may favor kinetically controlled products (products formed faster). The solvent influences the solvation of reactants and intermediates, affecting reaction rates and selectivity. The choice of nucleophile, base, or electrophile significantly impacts the outcome. Stronger bases favor elimination reactions, while weaker bases favor substitution.
4. Kinetic vs. Thermodynamic Control: A Crucial Distinction
Kinetic control refers to the formation of products based on reaction rates. The faster reaction pathway leading to the kinetic product is favored. Thermodynamic control refers to the formation of products based on stability. The more stable product, even if formed slower, will be the major product under thermodynamic control. Temperature plays a major role in determining kinetic vs. thermodynamic control.
Predicting the Major Product: A Step-by-Step Approach
Let's illustrate the prediction process with examples:
Example 1: SN1 vs. SN2
Consider the reaction of 2-bromo-2-methylpropane with methanol. The tertiary alkyl halide favors SN1 due to the highly stable tertiary carbocation intermediate. Therefore, the major product is 2-methoxy-2-methylpropane. In contrast, the reaction of methyl bromide with methanol would favor SN2 due to the absence of steric hindrance, resulting in methyl methyl ether as the major product.
Example 2: E1 vs. E2
The reaction of 2-bromo-2-methylbutane with a strong base like potassium tert-butoxide will primarily undergo E2 elimination. Due to steric hindrance, Hofmann elimination might be favored, leading to the less substituted alkene as the major product. However, with a weaker base or under conditions favoring E1, the Zaitsev product (more substituted alkene) would be favored.
Example 3: Electrophilic Aromatic Substitution
Consider the nitration of toluene. The methyl group is an electron-donating group, directing the nitronium ion (NO2+) to the ortho and para positions. The para product is usually the major product due to less steric hindrance compared to the ortho product.
Advanced Considerations: Regioselectivity and Stereoselectivity
Regioselectivity refers to the preferential formation of one constitutional isomer over another. Markovnikov's rule, Zaitsev's rule, and directing effects in electrophilic aromatic substitution are examples of regioselectivity.
Stereoselectivity refers to the preferential formation of one stereoisomer over another. SN2 reactions exhibit stereoselectivity with inversion of configuration. E2 reactions show stereoselectivity with a preference for anti-periplanar geometry.
Conclusion: Mastering the Art of Prediction
Accurately predicting the major product of an organic reaction is a multifaceted skill that combines a thorough understanding of reaction mechanisms, factors influencing reaction pathways, and the ability to weigh the relative importance of steric effects, electronic effects, and reaction conditions. By systematically analyzing these elements, you can confidently predict the outcome of diverse organic reactions. Remember, practice is key; the more examples you work through, the more proficient you'll become in this crucial aspect of organic chemistry. This comprehensive guide provides a strong foundation for this challenging yet rewarding aspect of the subject. Continuous learning and refinement of your understanding will ultimately lead to accurate predictions and a deeper understanding of the intricacies of organic reactivity.
Latest Posts
Latest Posts
-
Which Of The Following Are Grocery Stores Allowed To Do
Mar 10, 2025
-
Under Ctpat Your Carrier Is Required To Follow
Mar 10, 2025
-
Select The True Statements About Hydrocarbons
Mar 10, 2025
-
Which Mirror Provides A Wider Perspective To Minimize Blind Spots
Mar 10, 2025
-
What Is The First Step Of Evasive Steering
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Predict The Major Product Of The Following Reaction. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
