Lysosomes Are Membrane-bound Vesicles That Arise From The
Holbox
Mar 14, 2025 · 6 min read
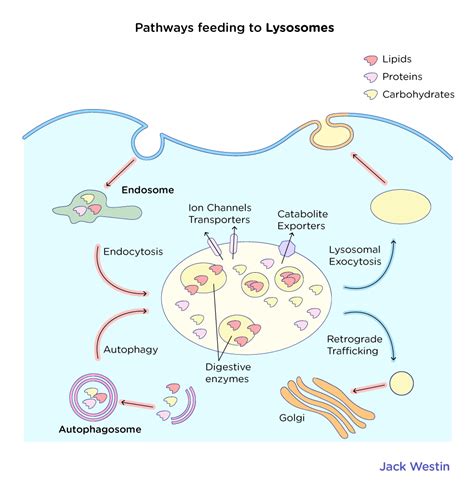
Table of Contents
Lysosomes: Membrane-Bound Vesicles Originating from the Golgi Apparatus – A Deep Dive
Lysosomes, often described as the cell's recycling centers or waste disposal units, are crucial organelles playing a vital role in maintaining cellular health and homeostasis. These membrane-bound vesicles, originating primarily from the Golgi apparatus, are filled with a diverse array of hydrolytic enzymes capable of breaking down a wide spectrum of biological macromolecules. Their precise function extends far beyond simple waste disposal, impacting cellular processes such as nutrient acquisition, signal transduction, and even programmed cell death. This article will delve deep into the intricacies of lysosomes, exploring their origin, composition, function, and the implications of their dysfunction.
The Genesis of Lysosomes: From Golgi to Functional Organelle
The journey of a lysosome begins within the vast and dynamic network of the Golgi apparatus. While the precise mechanisms are still under investigation, the generally accepted pathway involves the budding of vesicles from the trans Golgi network (TGN). These nascent vesicles are packed with newly synthesized lysosomal hydrolases – enzymes specifically designed to function in the acidic environment of the lysosome. These enzymes, including proteases, nucleases, lipases, glycosidases, and phosphatases, are tagged with a unique molecular marker, mannose-6-phosphate (M6P), during their synthesis. This M6P tag acts as a "zip code," directing the enzymes to their destined location within the lysosome.
The Role of M6P Receptors in Lysosome Targeting
The M6P receptor, a transmembrane protein located within the TGN, plays a crucial role in recognizing and binding to the M6P-tagged hydrolases. This interaction triggers the formation of clathrin-coated vesicles, encapsulating the enzymes and initiating their transport towards the lysosome. Once these vesicles reach the endosome – an intermediate compartment in the endocytic pathway – the acidic pH triggers the dissociation of the M6P receptor from the hydrolases. The receptor is then recycled back to the TGN, while the hydrolases are ultimately delivered to the lysosome. This intricate process ensures the precise targeting of these crucial enzymes to their designated compartment.
Other Pathways Contributing to Lysosome Biogenesis
While the Golgi-derived pathway is the primary route for lysosome formation, other pathways also contribute to their biogenesis. Autophagy, a process of self-digestion, plays a critical role in delivering cytoplasmic components to the lysosome for degradation. During autophagy, portions of the cytoplasm are enclosed within double-membrane vesicles called autophagosomes. These autophagosomes then fuse with lysosomes, forming autolysosomes, where the enclosed contents are broken down. Furthermore, endocytosis, the process of internalizing extracellular materials, also delivers substances to the lysosome for degradation.
The Internal Milieu of Lysosomes: An Acidic Environment for Enzymatic Activity
The lysosomal lumen, the internal space of the lysosome, is characterized by its remarkably acidic pH, typically ranging from 4.5 to 5.0. This acidic environment is crucial for the optimal activity of the lysosomal hydrolases, many of which are only active at low pH. The maintenance of this acidic pH is primarily achieved by the action of a vacuolar-type H+-ATPase (V-ATPase), a proton pump embedded in the lysosomal membrane. This pump actively transports protons (H+) from the cytoplasm into the lysosomal lumen, creating and maintaining the necessary acidic environment.
The Diverse Enzymatic Arsenal within Lysosomes
The lysosome's arsenal of hydrolytic enzymes is remarkably diverse, reflecting the wide range of substrates it is capable of degrading. These enzymes can break down almost all types of biological macromolecules, including:
- Proteins: Proteases degrade proteins into their constituent amino acids.
- Nucleic acids: Nucleases break down DNA and RNA into nucleotides.
- Lipids: Lipases hydrolyze lipids into fatty acids and glycerol.
- Carbohydrates: Glycosidases break down complex carbohydrates into simpler sugars.
- Phospholipids: Phosphatases hydrolyze phospholipids.
This enzymatic diversity enables the lysosome to effectively recycle and degrade a broad spectrum of cellular components and ingested materials.
The Multifaceted Functions of Lysosomes: Beyond Waste Disposal
The role of lysosomes extends far beyond their initial characterization as simple waste disposal units. Their functions are crucial for maintaining cellular health and participate in a variety of essential cellular processes:
1. Cellular Recycling and Nutrient Acquisition:
Lysosomes play a vital role in cellular recycling through autophagy and endocytosis. By breaking down unwanted or damaged cellular components, they release valuable building blocks like amino acids, nucleotides, and fatty acids, which can be reused for the synthesis of new molecules. This recycling process is crucial for cellular efficiency and resource management.
2. Defense Against Pathogens:
Lysosomes are key players in the innate immune response against pathogens. They engulf and degrade invading bacteria and viruses through a process called phagocytosis. The acidic environment and hydrolytic enzymes within the lysosome effectively eliminate the pathogens, preventing their spread and protecting the cell from infection.
3. Signal Transduction and Cellular Communication:
Emerging research indicates that lysosomes are not merely passive players but also active participants in cellular signaling pathways. They release signaling molecules that can influence cellular processes like cell growth, differentiation, and apoptosis (programmed cell death). Lysosomal membrane proteins have been shown to interact with other cellular signaling molecules, mediating cellular responses to stimuli.
4. Programmed Cell Death (Apoptosis):
Lysosomes play a role in programmed cell death, a crucial process for maintaining tissue homeostasis and eliminating damaged or unwanted cells. The release of lysosomal enzymes into the cytoplasm triggers a cascade of events leading to cellular dismantling and death. This controlled cell death is essential for development and preventing the accumulation of damaged or cancerous cells.
Lysosomal Dysfunction and Associated Diseases
Dysfunction in lysosomal function can have severe consequences, leading to a group of inherited disorders known as lysosomal storage disorders (LSDs). These diseases result from defects in one or more lysosomal enzymes, leading to the accumulation of undigested substrates within the lysosome. This accumulation can disrupt cellular function and lead to a variety of clinical manifestations, depending on the specific enzyme defect and the accumulated substrate. Some well-known LSDs include:
- Gaucher disease: Deficiency in glucocerebrosidase, leading to the accumulation of glucosylceramide.
- Tay-Sachs disease: Deficiency in β-hexosaminidase A, leading to the accumulation of gangliosides.
- Hurler syndrome: Deficiency in α-L-iduronidase, leading to the accumulation of glycosaminoglycans.
These diseases highlight the crucial role lysosomes play in maintaining cellular homeostasis and the profound impact of their dysfunction on human health.
Future Research Directions: Unraveling the Complexities of Lysosomes
Despite extensive research, many aspects of lysosomal biology remain to be fully elucidated. Future research will continue to focus on:
- Uncovering the precise mechanisms of lysosome biogenesis and regulation: This includes a deeper understanding of the trafficking pathways involved in delivering enzymes to the lysosome and the regulatory mechanisms controlling lysosomal function.
- Investigating the role of lysosomes in various cellular processes: This involves further exploration of their involvement in signal transduction, cell growth, differentiation, and apoptosis.
- Developing novel therapeutic strategies for lysosomal storage disorders: This includes research into enzyme replacement therapy, gene therapy, and pharmacological chaperones to alleviate the symptoms and improve the quality of life for individuals affected by these debilitating diseases.
- Exploring the link between lysosomal dysfunction and other age-related diseases: Emerging evidence suggests that lysosomal dysfunction may contribute to the pathogenesis of neurodegenerative diseases, cancer, and other age-related disorders.
The study of lysosomes continues to reveal exciting new insights into their complexity and importance. As our understanding grows, new therapeutic avenues may be discovered, offering hope for individuals affected by lysosomal disorders and enhancing our understanding of fundamental cellular processes. The seemingly humble lysosome truly stands as a remarkable organelle, pivotal to cellular health and a fascinating subject for ongoing scientific investigation.
Latest Posts
Latest Posts
-
Managers Must Recognize That Motivating Individuals Today Requires
Mar 14, 2025
-
Drag Each Label To The Appropriate Location On The Flowchart
Mar 14, 2025
-
The Manufacturing Overhead Account Is Debited When
Mar 14, 2025
-
Test Your Basic Knowledge About Clotting Factors And Anticoagulants
Mar 14, 2025
-
1 In 8 Customers Will Not Renew
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Lysosomes Are Membrane-bound Vesicles That Arise From The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
