In The Molecule Bri Which Atom Is The Negative Pole
Holbox
Mar 16, 2025 · 5 min read
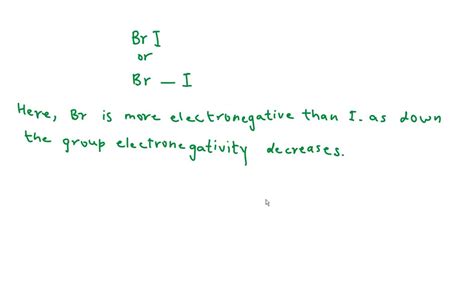
Table of Contents
In the BRI Molecule, Which Atom is the Negative Pole? Understanding Molecular Polarity
Determining the negative pole in a molecule like BRI (assuming this refers to a specific molecule and not an acronym needing further clarification) hinges on understanding molecular polarity. This concept arises from the difference in electronegativity between atoms within a molecule. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. Let's delve into the details.
Understanding Electronegativity and its Role in Molecular Polarity
Electronegativity is a fundamental property in chemistry, influencing how electrons are shared or transferred between atoms. Atoms with high electronegativity strongly attract electrons, while those with low electronegativity hold onto electrons less tightly. The difference in electronegativity between atoms within a molecule dictates the molecule's polarity.
The Electronegativity Scale
The Pauling scale is the most commonly used electronegativity scale. Fluorine (F) is assigned the highest value (4.0), and the values decrease as you move down and to the left on the periodic table. This scale provides a relative comparison of electronegativity between different elements.
Polar vs. Nonpolar Bonds
-
Polar Bonds: When two atoms with significantly different electronegativities form a bond, the electrons are unequally shared. The atom with higher electronegativity attracts the electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. This is a polar covalent bond. Think of water (H₂O) as a prime example; oxygen is significantly more electronegative than hydrogen, leading to a polar O-H bond.
-
Nonpolar Bonds: When two atoms with similar electronegativities bond, the electrons are shared relatively equally, resulting in a nonpolar covalent bond. For example, the bond between two identical atoms (e.g., Cl-Cl in chlorine gas) is always nonpolar.
Determining Molecular Polarity: Beyond Individual Bonds
Even if a molecule contains polar bonds, the overall molecule might be nonpolar. This depends on the molecule's geometry and the vectorial addition of the individual bond dipoles.
Molecular Geometry and Dipole Moments
Molecular geometry, determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory, describes the three-dimensional arrangement of atoms in a molecule. Each polar bond has a dipole moment, represented by a vector pointing from the positive to the negative pole. If these dipole moments cancel each other out due to symmetry in the molecular geometry, the overall molecule is nonpolar.
Examples illustrating the effect of Geometry
-
Carbon Dioxide (CO₂): While the C=O bonds are polar (oxygen is more electronegative), the linear geometry of CO₂ means the dipole moments of the two C=O bonds point in opposite directions and cancel each other out. Therefore, CO₂ is a linear, nonpolar molecule.
-
Water (H₂O): The O-H bonds are polar, and the bent geometry prevents the dipole moments from canceling. The resultant dipole moment gives water its overall polarity, making it a polar molecule.
Applying the Concepts to the BRI Molecule (Hypothetical Example)
Since "BRI" isn't a standard chemical formula, let's assume it represents a hypothetical molecule to illustrate the process. Let's imagine BRI has the following structure: B-R-I, where B, R, and I represent different atoms with different electronegativities.
To determine the negative pole, we need:
-
Electronegativity Values: Obtain the electronegativity values for atoms B, R, and I from a reliable source like a periodic table with electronegativity values.
-
Bond Polarity: Compare the electronegativity values of each pair of bonded atoms (B-R and R-I). The atom with the higher electronegativity will have a partial negative charge (δ-).
-
Molecular Geometry: Determine the geometry of the BRI molecule using VSEPR theory. This will be crucial in determining if the individual bond dipoles cancel out. Linear, trigonal planar, tetrahedral, etc., each has a different impact on overall polarity.
-
Vectorial Addition of Dipole Moments: If the molecule is not linear and symmetrical, we must consider the vectorial addition of the bond dipoles. This is usually represented using arrows to visualize the overall dipole moment. The atom(s) at the end of the resultant arrow will indicate the negative pole.
Example Scenario:
Let's assume (hypothetically) that:
- B has electronegativity 2.0
- R has electronegativity 2.5
- I has electronegativity 3.0
In this scenario:
- The B-R bond would be slightly polar, with R being slightly negative (δ-).
- The R-I bond would be more polar, with I being strongly negative (δ-).
If the molecule is linear, the negative pole would be I because it has a significantly higher electronegativity than both B and R. However, if the molecule has a bent or other asymmetrical geometry, the negative pole might not simply align with the atom possessing the highest electronegativity. The resultant dipole moment from vector addition would determine the exact location of the negative pole.
Importance of Identifying the Negative Pole
Understanding molecular polarity and identifying the negative pole is crucial in various fields:
-
Solubility: Polar molecules tend to dissolve in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents.
-
Biological Interactions: Many biological interactions rely on the polarity of molecules. For instance, the polarity of water is critical for life processes.
-
Chemical Reactions: Molecular polarity influences reactivity. Polar molecules are more likely to participate in certain types of reactions compared to nonpolar molecules.
-
Spectroscopy: Techniques like infrared (IR) spectroscopy can be used to determine molecular polarity based on the absorption of infrared radiation.
-
Material Science: Molecular polarity plays a vital role in the properties of materials, influencing their behavior in applications like polymers and coatings.
Conclusion: A Systematic Approach to Determining Polarity
Determining the negative pole in a molecule like BRI requires a systematic approach involving understanding electronegativity, bond polarity, molecular geometry, and the vectorial addition of dipole moments. While this example illustrated a hypothetical molecule, the fundamental principles remain the same for any molecule, regardless of its complexity. Remember to always refer to reliable resources for electronegativity values and apply the concepts of VSEPR theory to determine the three-dimensional structure of the molecule accurately. This process is crucial for understanding the chemical and physical properties of molecules and their behavior in different environments.
Latest Posts
Latest Posts
-
Draw All Resonance Structures For The Nitryl Fluoride Molecule No2f
Mar 16, 2025
-
Property Rights Are Important Because They
Mar 16, 2025
-
Host Range Is Limited By The
Mar 16, 2025
-
Draw The Major Organic Product Formed In The Reaction
Mar 16, 2025
-
Lymph Nodes Do All Of The Following Except
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about In The Molecule Bri Which Atom Is The Negative Pole . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
