If Calcium Ions Each Of Which Has A Charge Of
Holbox
Mar 24, 2025 · 6 min read
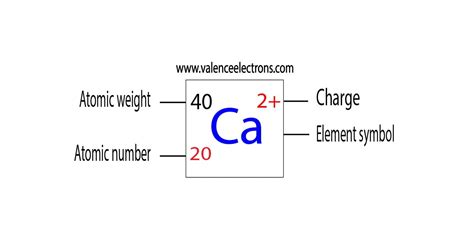
Table of Contents
- If Calcium Ions Each Of Which Has A Charge Of
- Table of Contents
- The Fascinating World of Calcium Ions: Their Charge and Biological Significance
- The +2 Charge: A Foundation for Interaction
- The Impact of Charge on Calcium's Biological Functions
- Calcium Homeostasis: A Delicate Balancing Act
- Dysregulation of Calcium: Implications for Health
- Future Directions and Research
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
The Fascinating World of Calcium Ions: Their Charge and Biological Significance
Calcium ions (Ca²⁺), each carrying a +2 charge, are ubiquitous in biological systems, playing a crucial role in a vast array of cellular processes. Understanding their fundamental properties, specifically their charge and how it influences their interactions, is key to appreciating their immense biological importance. This article delves deep into the world of calcium ions, exploring their charge, its implications for their behavior, and their multifaceted roles in maintaining life.
The +2 Charge: A Foundation for Interaction
The +2 charge of a calcium ion is a defining characteristic that governs its interactions with other molecules. This double positive charge makes it highly reactive, readily attracting negatively charged molecules or regions within molecules. This electrostatic attraction is the basis for calcium's involvement in numerous biological processes, including:
-
Signal Transduction: Calcium ions act as crucial second messengers in many signaling pathways. Their influx into a cell, often triggered by external stimuli, triggers a cascade of events leading to specific cellular responses. The +2 charge allows for highly specific binding to proteins involved in these pathways.
-
Enzyme Regulation: Many enzymes require calcium ions for their activity. The binding of Ca²⁺ to these enzymes often induces conformational changes, activating or inhibiting their catalytic function. The strong electrostatic interaction ensures tight and specific binding.
-
Muscle Contraction: The interaction of calcium ions with proteins in muscle cells is fundamental to muscle contraction. The +2 charge facilitates the binding of calcium to troponin, a protein that regulates the interaction between actin and myosin, the contractile proteins in muscle.
-
Bone Formation: Calcium ions are a major structural component of bones and teeth. The strong ionic bonds formed between calcium and phosphate ions create the hard, mineralized matrix that provides structural support to the body. The +2 charge is crucial for the formation and stability of these mineral deposits.
-
Blood Clotting: Calcium ions are essential for blood coagulation. They act as cofactors in several steps of the coagulation cascade, facilitating the formation of blood clots and preventing excessive bleeding. The +2 charge mediates interactions between different clotting factors.
The Impact of Charge on Calcium's Biological Functions
The +2 charge of the calcium ion is not merely a static property; it dynamically influences its behavior and interactions within the cellular environment. Several factors contribute to this dynamic influence:
-
Concentration Gradients: The concentration of calcium ions is tightly regulated within cells and across cell membranes. Maintaining a steep concentration gradient, with significantly lower intracellular calcium concentrations compared to extracellular levels, is essential for calcium's role as a signaling molecule. This gradient relies on the charge of the ion, and its movement across membranes is highly regulated by specific ion channels and pumps.
-
Binding Affinity: The +2 charge directly impacts the binding affinity of calcium to various proteins and molecules. Higher charges generally lead to stronger binding, but the specificity of binding is also determined by other factors such as the three-dimensional structure of the binding site. This selectivity ensures that calcium interacts with specific targets, mediating specific cellular responses.
-
Chelation: Calcium ions can bind to chelating agents, molecules with multiple electron donor groups that form stable complexes with metal ions. These chelators can influence the availability of free calcium ions, modulating calcium's biological activity. The +2 charge makes calcium highly susceptible to chelation.
-
Interaction with other Ions: The presence of other ions in the cellular environment can influence the behavior of calcium ions. For example, the presence of magnesium ions (Mg²⁺), which also carry a +2 charge, can compete with calcium for binding sites, affecting the efficiency of calcium-mediated processes.
Calcium Homeostasis: A Delicate Balancing Act
Maintaining appropriate calcium levels within cells and throughout the body, a process known as calcium homeostasis, is crucial for health and survival. This intricate process involves a complex interplay of several mechanisms, all influenced by the unique properties of the calcium ion:
-
Calcium Channels and Pumps: Specialized proteins embedded in cell membranes regulate the influx and efflux of calcium ions. Calcium channels allow the entry of calcium into cells, while calcium pumps actively transport calcium out of cells or into intracellular stores. The charge of the calcium ion necessitates these energy-dependent pumps to overcome the electrostatic forces that oppose its movement.
-
Intracellular Calcium Stores: Cells possess specialized organelles, such as the endoplasmic reticulum and sarcoplasmic reticulum, which act as reservoirs for calcium ions. These stores release calcium upon stimulation, rapidly increasing intracellular calcium concentration and triggering downstream signaling events. The charge of calcium ions plays a crucial role in how they are stored and released from these organelles.
-
Calcium-Binding Proteins: A variety of calcium-binding proteins, including calmodulin, troponin, and parvalbumin, buffer changes in intracellular calcium concentrations and mediate calcium's effects on cellular processes. These proteins have specific binding sites with high affinity for calcium ions, owing to the +2 charge of the ion.
-
Hormonal Regulation: Hormones such as parathyroid hormone (PTH) and calcitonin regulate calcium levels in the blood by affecting calcium absorption in the gut, calcium excretion by the kidneys, and bone resorption and formation. The +2 charge of calcium plays a central role in the actions of these hormones.
Dysregulation of Calcium: Implications for Health
Disruptions in calcium homeostasis can have profound implications for health. Hypocalcemia, a deficiency of calcium in the blood, can lead to muscle weakness, tetany, and cardiac arrhythmias. Conversely, hypercalcemia, an excess of calcium in the blood, can cause kidney stones, constipation, and neurological symptoms.
Several diseases are associated with calcium dysregulation, including:
-
Osteoporosis: This debilitating disease is characterized by decreased bone density, making bones fragile and prone to fractures. It's often associated with impaired calcium homeostasis.
-
Cardiovascular Diseases: Abnormal calcium handling in the heart can contribute to various cardiac arrhythmias and heart failure. The +2 charge of calcium is crucial for normal heart function, and its dysregulation can lead to severe consequences.
-
Neurological Disorders: Calcium dysregulation can impact neuronal excitability and synaptic transmission, potentially contributing to neurological disorders such as epilepsy and Alzheimer's disease.
Future Directions and Research
Despite the vast knowledge accumulated about calcium ions and their biological roles, ongoing research continues to unravel the intricacies of calcium's influence on cellular processes. Future research will likely focus on:
-
Developing new therapies targeting calcium dysregulation: Advances in understanding calcium signaling pathways and their dysregulation in various diseases are paving the way for developing novel therapeutic strategies.
-
Exploring the role of calcium in complex biological processes: The role of calcium in development, aging, and cancer is an active area of research.
-
Improving techniques for monitoring calcium dynamics: Advanced imaging techniques and biosensors are being developed to visualize and quantify calcium fluxes within cells, offering valuable insights into cellular processes.
Conclusion
The +2 charge of the calcium ion is not merely a physical property; it is a fundamental determinant of its biological activity. This charge underpins calcium's roles in numerous essential cellular processes, from signal transduction and muscle contraction to bone formation and blood clotting. Maintaining calcium homeostasis is crucial for health, and dysregulation of calcium levels can have severe consequences. Continued research promises to further illuminate the multifaceted roles of calcium ions in biological systems, offering opportunities for developing new diagnostic tools and therapies for a variety of diseases. The journey of understanding the calcium ion is far from over; it remains a compelling area of investigation, promising exciting discoveries in the years to come.
Latest Posts
Latest Posts
-
Why Should You Curate A Buyer Persona Story
Mar 26, 2025
-
Drag The Appropriate Labels To Their Respective Targets Resethelp
Mar 26, 2025
-
The Accompanying Graph Depicts An Economy In The
Mar 26, 2025
-
A Bottle Of Milk Is Taken Out Of A Refrigerator
Mar 26, 2025
-
Do Black People Have An Extra Muscle In Their Leg
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about If Calcium Ions Each Of Which Has A Charge Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
