Identify What A Coffee Cup Calorimeter Measures
Holbox
Mar 21, 2025 · 7 min read
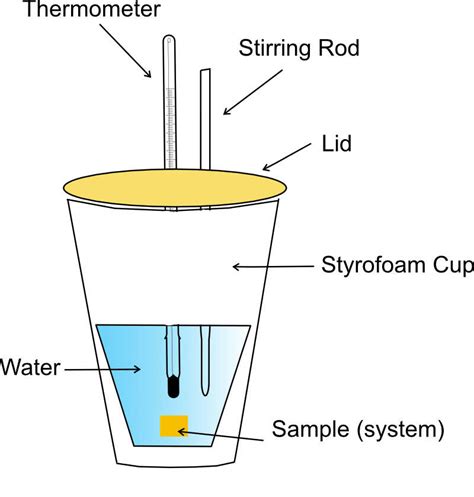
Table of Contents
Identifying What a Coffee Cup Calorimeter Measures: A Comprehensive Guide
A coffee cup calorimeter, also known as a constant-pressure calorimeter, is a simple yet essential tool in chemistry and thermodynamics used to measure the heat transfer during a chemical reaction or physical process occurring in solution. Understanding exactly what it measures and how it does so is crucial for accurate interpretation of experimental data. This article will delve into the intricacies of coffee cup calorimetry, exploring its applications, limitations, and the fundamental principles behind its operation.
What Does a Coffee Cup Calorimeter Measure?
At its core, a coffee cup calorimeter measures the heat exchanged between a system (the reaction mixture) and its surroundings (the calorimeter itself and the surrounding air). This heat exchange, expressed as heat capacity, directly relates to the change in enthalpy (ΔH) of the reaction, provided the reaction is carried out under constant pressure conditions (as is typically the case with a coffee cup calorimeter). The calorimeter itself doesn't directly measure enthalpy; instead, it measures the temperature change resulting from the heat transfer, which is then used to calculate the enthalpy change.
The Crucial Role of Heat Transfer
The principle behind a coffee cup calorimeter lies in the law of conservation of energy: energy cannot be created or destroyed, only transferred or converted from one form to another. In a chemical reaction, the energy released or absorbed manifests as heat. A coffee cup calorimeter works by isolating the reaction system to minimize heat exchange with the outside environment. Any temperature change within the calorimeter is thus primarily due to the heat released or absorbed by the reaction.
Enthalpy Change (ΔH): The Primary Measured Parameter
The ultimate goal of using a coffee cup calorimeter is to determine the enthalpy change (ΔH) of a reaction. Enthalpy is a thermodynamic state function representing the total heat content of a system at constant pressure. A positive ΔH indicates an endothermic reaction (heat is absorbed from the surroundings), while a negative ΔH indicates an exothermic reaction (heat is released to the surroundings). The magnitude of ΔH reflects the amount of heat exchanged during the reaction.
How the Coffee Cup Calorimeter Works: A Step-by-Step Explanation
-
Setup: The calorimeter consists of two nested Styrofoam cups (providing insulation to minimize heat loss to the environment), a thermometer to monitor temperature changes, and a stirrer to ensure uniform temperature distribution. The reaction mixture is prepared and placed inside the inner cup.
-
Reaction Initiation: The reaction is initiated, either by mixing reactants or adding a catalyst. The thermometer is used to record the initial temperature (T<sub>i</sub>) before the reaction begins.
-
Temperature Monitoring: The temperature of the reaction mixture is monitored over time. The temperature will either increase (exothermic reaction) or decrease (endothermic reaction) as the reaction proceeds. The highest or lowest temperature (T<sub>f</sub>) reached is recorded.
-
Data Analysis: The temperature change (ΔT = T<sub>f</sub> - T<sub>i</sub>) is used to calculate the heat exchanged (q) using the following equation:
q = mcΔT
where:
- q = heat exchanged (in Joules)
- m = mass of the solution (in grams)
- c = specific heat capacity of the solution (usually assumed to be close to the specific heat capacity of water, 4.18 J/g°C)
- ΔT = change in temperature (°C)
-
Enthalpy Calculation: Once the heat exchanged (q) is calculated, it represents the enthalpy change (ΔH) of the reaction if the reaction is carried out under constant pressure conditions. To express the enthalpy change on a molar basis (ΔH<sub>m</sub>), the heat exchanged is divided by the number of moles of the limiting reactant:
ΔH<sub>m</sub> = q / n
where:
- ΔH<sub>m</sub> = molar enthalpy change (J/mol)
- n = number of moles of the limiting reactant
Factors Affecting Accuracy of Measurements: Sources of Error
While the coffee cup calorimeter is a simple and effective tool, several factors can affect the accuracy of the measurements obtained. It’s crucial to be aware of these limitations:
1. Heat Loss to the Surroundings: Imperfect Insulation
The Styrofoam cups provide insulation, but they are not perfect insulators. Some heat will inevitably be lost to the surroundings, leading to an underestimation of the magnitude of ΔH for exothermic reactions and an overestimation for endothermic reactions. This error can be minimized by using well-insulated cups and performing the experiment quickly.
2. Heat Capacity of the Calorimeter: The "Calorimeter Constant"
The equation q = mcΔT assumes that all the heat released or absorbed by the reaction goes into heating or cooling the solution. However, some heat is absorbed by the calorimeter itself (cups, thermometer, stirrer). This can be accounted for by determining the calorimeter constant (C<sub>cal</sub>), which represents the heat capacity of the calorimeter. The corrected equation becomes:
q<sub>rxn</sub> = -(q<sub>sol</sub> + C<sub>cal</sub>ΔT)
Where q<sub>rxn</sub> is the heat of reaction, and q<sub>sol</sub> represents the heat absorbed or released by the solution. Determining C<sub>cal</sub> often requires a separate calibration experiment.
3. Incomplete Reaction: Non-quantitative conversion
The calculations assume that the reaction goes to completion. If the reaction does not reach completion, the calculated ΔH will be lower than the actual value for exothermic reactions and higher for endothermic reactions.
4. Heat of Mixing and Dilution: Non-negligible effects
The process of mixing the reactants and dissolving them in the solvent can itself produce a small amount of heat. This heat of mixing or dilution should ideally be considered for more accurate results.
5. Specific Heat Capacity Assumption: Beyond Water
The equation often assumes the specific heat capacity of the solution is the same as that of water. This is a reasonable approximation for dilute aqueous solutions but can introduce errors when dealing with concentrated solutions or non-aqueous solvents. The actual specific heat capacity of the solution should be used for greater accuracy.
6. Temperature Measurement Errors: Instrumental limitations
Errors in the measurement of the initial and final temperatures using the thermometer will directly impact the calculated ΔT and subsequently, the enthalpy change. Using a high-precision thermometer is crucial.
Advanced Applications and Modifications: Beyond Basic Calorimetry
While the basic coffee cup calorimeter is widely used for introductory chemistry experiments, several modifications and advanced applications exist:
1. Bomb Calorimetry: Constant-Volume Calorimetry
For reactions involving gases or significant volume changes, a bomb calorimeter is employed. This type of calorimeter operates at constant volume, measuring the change in internal energy (ΔU) rather than enthalpy. Bomb calorimeters are often used for combustion reactions.
2. Isoperibol Calorimetry: Precise temperature control
Isoperibol calorimeters use a sophisticated design with a controlled temperature jacket around the reaction vessel, leading to more precise and accurate temperature control. This reduces the influence of heat exchange with the environment.
3. Reaction Calorimetry: Monitoring reactions over time
Reaction calorimetry involves measuring the heat flow during a reaction as a function of time. This technique provides more comprehensive information about the reaction kinetics and thermodynamics.
4. Microcalorimetry: High sensitivity
Microcalorimeters use extremely sensitive sensors to measure the heat flow from very small samples. This is useful for studying biological systems and other delicate reactions.
Conclusion: A Versatile Tool in Thermodynamics
The coffee cup calorimeter, despite its simple design, serves as a powerful tool for understanding the heat transfer associated with chemical reactions and physical processes. By carefully considering the sources of error and employing appropriate techniques, accurate measurements of enthalpy changes can be obtained. Understanding the limitations of this method and the potential for improvement through modifications and advanced techniques allows researchers to effectively utilize this essential tool for various thermodynamic studies. The information obtained from a coffee cup calorimeter contributes significantly to our understanding of reaction energetics and enables quantitative analysis of chemical and physical changes. This makes it an invaluable apparatus in chemistry education and research alike.
Latest Posts
Latest Posts
-
A Price Setter Company Will Use More
Mar 22, 2025
-
A Major Purpose Of Cost Accounting Is To
Mar 22, 2025
-
Fetal And Neonatal Pharmacology For The Advanced Practice Nurse
Mar 22, 2025
-
When Must Emergency Preparedness Drills Be Conducted
Mar 22, 2025
-
A Business Message Is Complete If It
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Identify What A Coffee Cup Calorimeter Measures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
