Identify The Components Contained In Each Of The Following Lipids.
Holbox
Mar 12, 2025 · 7 min read
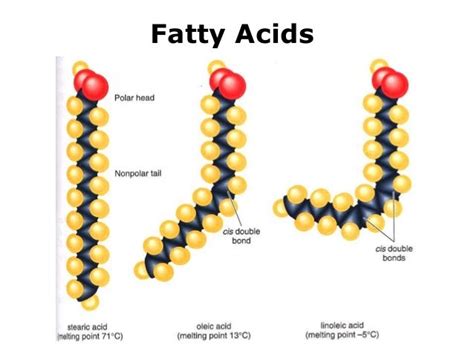
Table of Contents
- Identify The Components Contained In Each Of The Following Lipids.
- Table of Contents
- Identify the Components Contained in Each of the Following Lipids
- 1. Fatty Acids: The Building Blocks of Many Lipids
- Components of a Fatty Acid:
- 2. Triglycerides: The Primary Energy Storage Lipids
- Components of a Triglyceride:
- 3. Phospholipids: The Essential Components of Cell Membranes
- Components of a Phospholipid:
- 4. Sphingolipids: Important Components of Cell Membranes and Signaling Molecules
- Components of a Sphingolipid:
- 5. Steroids: Diverse Lipids with Crucial Biological Roles
- Components of a Steroid:
- 6. Waxes: Protective Coatings and Energy Storage
- Components of a Wax:
- Lipid Classification Summary Table:
- The Importance of Understanding Lipid Components
- Latest Posts
- Latest Posts
- Related Post
Identify the Components Contained in Each of the Following Lipids
Lipids are a diverse group of naturally occurring organic compounds that are largely nonpolar and hydrophobic (water-insoluble). Unlike the other major classes of biological macromolecules (carbohydrates, proteins, and nucleic acids), lipids are not defined by a single type of monomeric unit. Instead, they are characterized by their solubility properties and their biological roles, which include energy storage, membrane structure, signaling, and insulation. This article will delve into the specific components of several key lipid classes.
1. Fatty Acids: The Building Blocks of Many Lipids
Fatty acids are the simplest lipids and serve as the building blocks for many complex lipids. They are long-chain carboxylic acids, typically containing between 4 and 36 carbon atoms. The hydrocarbon chain can be saturated (no double bonds), monounsaturated (one double bond), or polyunsaturated (two or more double bonds). The presence and location of double bonds significantly impact the fatty acid's properties.
Components of a Fatty Acid:
- Carboxylic acid group (-COOH): This polar head group is responsible for the slight acidity of fatty acids. It's the site where fatty acids can form ester bonds with other molecules to create more complex lipids.
- Hydrocarbon chain: This long, nonpolar tail is the hydrophobic part of the fatty acid. The length and degree of unsaturation of this chain determine the physical properties of the fatty acid and the lipids it forms. For example, saturated fatty acids pack tightly together, resulting in solid fats at room temperature, while unsaturated fatty acids have kinks in their chains due to the double bonds, leading to liquid oils at room temperature.
Examples:
- Palmitic acid (saturated): CH<sub>3</sub>(CH<sub>2</sub>)<sub>14</sub>COOH
- Oleic acid (monounsaturated): CH<sub>3</sub>(CH<sub>2</sub>)<sub>7</sub>CH=CH(CH<sub>2</sub>)<sub>7</sub>COOH
- Linoleic acid (polyunsaturated): CH<sub>3</sub>(CH<sub>2</sub>)<sub>4</sub>CH=CHCH<sub>2</sub>CH=CH(CH<sub>2</sub>)<sub>7</sub>COOH
2. Triglycerides: The Primary Energy Storage Lipids
Triglycerides, also known as triacylglycerols, are the most abundant form of lipids in the body and are the primary form of energy storage. They are composed of a glycerol molecule esterified to three fatty acids.
Components of a Triglyceride:
- Glycerol: A three-carbon alcohol with three hydroxyl (-OH) groups. Each hydroxyl group forms an ester bond with a fatty acid.
- Three fatty acids: These can be the same or different, saturated or unsaturated. The combination of fatty acids determines the physical properties (melting point, consistency) of the triglyceride. For instance, triglycerides rich in saturated fatty acids are solid at room temperature (fats), while those rich in unsaturated fatty acids are liquid (oils).
Understanding the esterification process: The formation of a triglyceride involves the dehydration reaction between the hydroxyl groups of glycerol and the carboxyl groups of three fatty acids. This results in the formation of three ester bonds and the release of three water molecules.
3. Phospholipids: The Essential Components of Cell Membranes
Phospholipids are the major structural components of cell membranes. They are amphipathic molecules, meaning they have both hydrophilic (water-loving) and hydrophobic (water-fearing) regions. This property allows them to form bilayers in aqueous environments, creating the foundation of cell membranes.
Components of a Phospholipid:
- Glycerol: Similar to triglycerides, phospholipids contain a glycerol backbone.
- Two fatty acids: These are usually attached to the first and second carbons of glycerol. These fatty acid tails are hydrophobic.
- Phosphate group: Attached to the third carbon of glycerol. The phosphate group is hydrophilic, carrying a negative charge.
- Polar head group: This is attached to the phosphate group. The polar head group can vary significantly, influencing the specific properties of different phospholipids (e.g., phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine). The head group is hydrophilic.
4. Sphingolipids: Important Components of Cell Membranes and Signaling Molecules
Sphingolipids are another important class of lipids found in cell membranes, especially in the nervous system. They are structurally distinct from phospholipids, based on a sphingosine backbone rather than glycerol.
Components of a Sphingolipid:
- Sphingosine: A long-chain amino alcohol.
- Fatty acid: Attached to the amino group of sphingosine via an amide linkage.
- Polar head group: Attached to the hydroxyl group of sphingosine. This group can be a variety of molecules, including phosphocholine (in sphingomyelins), sugars (in cerebrosides and gangliosides), or other functional groups. The head group's nature significantly affects the sphingolipid's function and properties.
5. Steroids: Diverse Lipids with Crucial Biological Roles
Steroids are a large and diverse group of lipids characterized by a four-ring hydrocarbon structure (three six-membered rings and one five-membered ring). They are involved in a wide range of biological functions, including hormone production and membrane fluidity regulation.
Components of a Steroid:
- Steroid nucleus: The four-ring hydrocarbon structure is the fundamental component of all steroids.
- Various side chains and functional groups: The specific side chains and functional groups attached to the steroid nucleus determine the specific properties and function of the steroid. For example, cholesterol has a hydroxyl group, while testosterone has a ketone group and a methyl group.
Examples of Steroids:
- Cholesterol: A crucial component of cell membranes, affecting membrane fluidity. It also serves as a precursor for the synthesis of other steroids, including steroid hormones.
- Testosterone: A male sex hormone crucial for development and maintenance of male characteristics.
- Estrogen: A group of female sex hormones essential for female reproductive function and secondary sexual characteristics.
- Cortisol: A glucocorticoid hormone involved in stress response and metabolism.
6. Waxes: Protective Coatings and Energy Storage
Waxes are esters of long-chain fatty acids and long-chain alcohols. They are generally hydrophobic and solid at room temperature.
Components of a Wax:
- Long-chain fatty acid: Typically contains 14 to 36 carbon atoms.
- Long-chain alcohol: Also typically contains 14 to 36 carbon atoms.
Lipid Classification Summary Table:
| Lipid Class | Main Components | Key Characteristics | Biological Function |
|---|---|---|---|
| Fatty Acids | Carboxylic acid group, hydrocarbon chain | Saturated, monounsaturated, or polyunsaturated | Building blocks of other lipids, energy source |
| Triglycerides | Glycerol, three fatty acids | Energy storage | Primary energy storage |
| Phospholipids | Glycerol, two fatty acids, phosphate group, polar head | Amphipathic, forms cell membranes | Major component of cell membranes |
| Sphingolipids | Sphingosine, fatty acid, polar head group | Membrane component, signaling molecules | Membrane structure, cell signaling |
| Steroids | Steroid nucleus, various side chains and functional groups | Four-ring structure | Hormones, membrane fluidity regulation |
| Waxes | Long-chain fatty acid, long-chain alcohol | Hydrophobic, solid at room temperature | Protective coatings, energy storage (in some organisms) |
The Importance of Understanding Lipid Components
Understanding the specific components of different lipid classes is crucial for several reasons:
- Understanding biological processes: The structure and composition of lipids directly influence their biological functions. For example, the degree of saturation in fatty acids affects membrane fluidity, and the polar head group of phospholipids dictates membrane interactions.
- Developing new drugs and therapies: Many drugs target lipid-based processes, such as cholesterol metabolism or inflammation. Understanding lipid composition is crucial for developing effective therapies.
- Nutritional science: The types of lipids consumed affect health outcomes. Understanding the composition of dietary fats is critical for maintaining a healthy diet.
- Diagnostics: Analysis of lipid profiles can be used to diagnose various diseases, including cardiovascular disease and metabolic disorders.
This detailed overview provides a comprehensive understanding of the diverse components of various lipid classes. Further research into specific lipid subtypes and their intricate roles within biological systems will continue to expand our knowledge and contribute to advancements in various fields, from medicine to nutrition.
Latest Posts
Latest Posts
-
How Many Cm Is 38 Inches
May 21, 2025
-
How Many Grams Is 15 Ounces
May 21, 2025
-
How Many Gallons Is 200 Liters
May 21, 2025
-
What Time Will It Be In 28 Minutes
May 21, 2025
-
690 Lbs In Stones And Pounds
May 21, 2025
Related Post
Thank you for visiting our website which covers about Identify The Components Contained In Each Of The Following Lipids. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.