How Would You Make The Following Compounds From N-benzylbenzamide
Holbox
Mar 13, 2025 · 6 min read
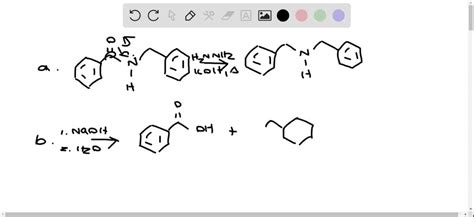
Table of Contents
Synthesizing Diverse Compounds from N-Benzylbenzamide: A Comprehensive Guide
N-Benzylbenzamide, a seemingly simple amide, serves as a surprisingly versatile starting material for the synthesis of a wide array of organic compounds. Its structure, featuring a benzene ring linked to a carbonyl group and a benzyl substituent on the nitrogen, provides multiple reactive sites amenable to various chemical transformations. This article explores several synthetic routes, detailing the mechanisms and conditions required to obtain diverse functional groups from this precursor. We'll delve into practical considerations, highlighting potential challenges and offering solutions for optimizing yields and purities.
1. Hydrolysis to Benzoic Acid and Benzylamine: A Classic Approach
The most straightforward transformation of N-benzylbenzamide involves its hydrolysis, yielding benzoic acid and benzylamine. This reaction, typically carried out under acidic or basic conditions, cleaves the amide bond.
Acidic Hydrolysis:
Strong mineral acids, such as concentrated hydrochloric acid or sulfuric acid, are employed. The reaction mechanism involves protonation of the carbonyl oxygen, followed by nucleophilic attack of water, leading to tetrahedral intermediate formation. Subsequent proton transfers and elimination steps yield the carboxylic acid and ammonium salt of benzylamine, which can then be neutralized to liberate the free amine. High temperatures and prolonged reaction times are often necessary to achieve complete conversion.
Basic Hydrolysis:
Strong bases like sodium hydroxide or potassium hydroxide in aqueous solution are commonly used. The mechanism involves deprotonation of the amide nitrogen, followed by nucleophilic attack of hydroxide ion on the carbonyl carbon. This leads to the formation of a tetrahedral intermediate, which collapses to yield the carboxylate anion and benzylamine. Careful control of the reaction temperature is crucial to avoid unwanted side reactions.
Considerations: Both acidic and basic hydrolysis can be effective, but the choice depends on the sensitivity of the products to acidic or basic conditions. Careful workup procedures are essential to isolate the benzoic acid and benzylamine efficiently. Purification techniques such as recrystallization or extraction may be required.
2. Reduction to N-Benzylbenzylamine: Accessing Secondary Amines
Reduction of the amide carbonyl group in N-benzylbenzamide offers access to N-benzylbenzylamine, a valuable secondary amine. Several reducing agents can achieve this transformation.
Lithium Aluminum Hydride (LAH): LAH is a potent reducing agent capable of reducing amides to amines. The reaction proceeds via a nucleophilic attack of the hydride ion on the carbonyl carbon, followed by a series of steps involving hydride transfer and protonation, ultimately leading to the formation of the secondary amine. LAH is highly reactive and requires anhydrous conditions. Careful addition and quenching protocols are essential to prevent vigorous reactions.
Borane (BH3): Borane, often in the form of borane-tetrahydrofuran complex, provides a milder alternative to LAH. It reacts with the amide similarly to LAH, but generally exhibits improved selectivity and fewer side reactions. The reaction typically requires elevated temperatures and longer reaction times.
Catalytic Hydrogenation: Catalytic hydrogenation using catalysts such as palladium on carbon (Pd/C) or Raney nickel can also reduce the amide to the amine, although higher pressures and temperatures might be needed compared to LAH or borane. This method offers the advantage of being relatively clean and mild.
Considerations: The choice of reducing agent depends on the desired level of reactivity and the tolerance of the substrate to different reaction conditions. Careful purification is necessary to separate the desired product from any unreacted starting material or byproducts.
3. Dehydration to N-Benzylnitrile: Exploring Nitrile Chemistry
Dehydration of N-benzylbenzamide yields N-benzylbenzonitrile. This transformation effectively removes a water molecule from the amide, converting the carbonyl group into a nitrile group.
Phosphorus Pentoxide (P2O5): P2O5 is a potent dehydrating agent that efficiently removes water from the amide. The reaction involves the formation of a complex between the amide and P2O5, followed by elimination of water. High temperatures are typically required for this reaction to proceed effectively.
Thionyl Chloride (SOCl2): SOCl2 reacts with the amide to form an intermediate imidoyl chloride, which subsequently undergoes dehydrochlorination to yield the nitrile. This method provides a relatively efficient and controlled way to synthesize nitriles from amides.
Considerations: Both methods can be effective, but P2O5 can be difficult to handle due to its hygroscopic nature. SOCl2 generates HCl as a byproduct, necessitating careful handling and workup procedures. Purification of the nitrile product may be required using techniques such as distillation or chromatography.
4. Hofmann Rearrangement: Accessing Amines with One Less Carbon Atom
The Hofmann rearrangement of N-benzylbenzamide is a valuable method for synthesizing amines with one carbon atom less than the starting material. The reaction involves treatment with a strong base (e.g., sodium hydroxide) followed by an electrophilic reagent such as bromine or N-bromosuccinimide. The mechanism involves the formation of an isocyanate intermediate that is then hydrolyzed to yield an amine. The resulting product will be benzyl amine.
Considerations: The reaction conditions are crucial for successful rearrangement. Careful control of temperature and stoichiometry is essential for optimal yield. Purification of the amine product may require techniques such as distillation or chromatography.
5. Schmidt Reaction: Another Route to Amines
The Schmidt reaction offers an alternative pathway to amines, employing hydrazoic acid (HN3) in the presence of a strong acid catalyst (e.g., sulfuric acid). This reaction leads to the formation of an amine and carbon dioxide. In the case of N-benzylbenzamide, the reaction generates benzylamine and carbon dioxide.
Considerations: Hydrazoic acid is highly toxic and explosive. The reaction requires careful handling and stringent safety precautions. The choice of acid catalyst can significantly influence the reaction outcome.
6. Reactions at the Benzyl Group: Exploring Further Functionality
The benzyl group in N-benzylbenzamide offers further synthetic opportunities. For instance, it can undergo:
-
Oxidation: Oxidation of the benzyl group can produce an aldehyde or carboxylic acid, depending on the oxidizing agent and reaction conditions. Reagents such as potassium permanganate or chromic acid can be employed.
-
Halogenation: The benzyl group can be halogenated using reagents such as N-bromosuccinimide (NBS) or chlorine.
-
Nitration: Nitration of the benzyl group introduces nitro functionality using a mixture of concentrated nitric and sulfuric acids.
Considerations: These reactions often require careful control of reaction conditions to prevent over-oxidation, over-halogenation, or multiple nitrations. Purification of the modified products might involve techniques such as recrystallization, chromatography, or distillation.
Conclusion
N-benzylbenzamide’s versatility as a synthetic building block is evident from the diverse transformations described. By carefully selecting appropriate reaction conditions and reagents, chemists can access a wide range of functional groups and molecular structures, including carboxylic acids, amines, nitriles, and modified benzyl derivatives. This detailed exploration underscores the importance of understanding reaction mechanisms and selecting optimal synthetic strategies for efficient and high-yielding transformations. Furthermore, careful consideration of safety protocols and purification techniques is paramount to successful synthesis. The information provided serves as a comprehensive guide for researchers working with N-benzylbenzamide and similar compounds, emphasizing the possibilities unlocked by this readily available starting material. Further exploration into specific reaction conditions, optimization strategies, and detailed reaction mechanisms for each transformation can be pursued based on specific research needs and synthetic goals.
Latest Posts
Latest Posts
-
Which Type Of Electron Is The Highest In Energy
Mar 13, 2025
-
Match The Description With The Concept Being Demonstrated
Mar 13, 2025
-
Darrel Needs To Fire A Manager
Mar 13, 2025
-
Both Hcl And Hbr Are Added To 8 Ethyldecan 5 Ol At 100oc
Mar 13, 2025
-
Creating New Pure Lines From Hybrid Plants Over Several Generations
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about How Would You Make The Following Compounds From N-benzylbenzamide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
