How Many Valence Electrons Does Na Have
Holbox
Mar 11, 2025 · 5 min read
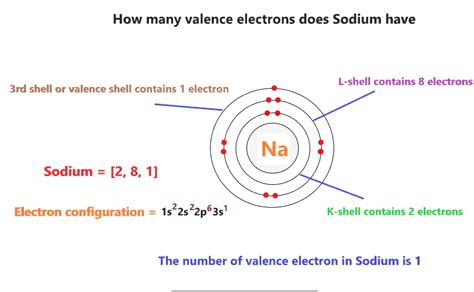
Table of Contents
- How Many Valence Electrons Does Na Have
- Table of Contents
- How Many Valence Electrons Does Na Have? A Deep Dive into Sodium's Electronic Structure
- Understanding Valence Electrons: The Key to Chemical Bonding
- Determining Sodium's Electronic Configuration
- Identifying Sodium's Valence Electrons
- Implications of Sodium's Single Valence Electron
- Sodium's Role in Biology and Industry
- Biological Significance:
- Industrial Applications:
- Beyond the Single Valence Electron: Exploring Related Concepts
- Conclusion: A Single Electron with Profound Implications
- Latest Posts
- Related Post
How Many Valence Electrons Does Na Have? A Deep Dive into Sodium's Electronic Structure
Sodium (Na), a ubiquitous element found in table salt and crucial for numerous biological processes, holds a key position in understanding basic chemistry concepts. One of the most fundamental aspects of an element’s reactivity and bonding behavior lies in its valence electrons – the electrons residing in the outermost shell. This article will comprehensively explore the number of valence electrons sodium possesses, delving into its electronic configuration, its implications for chemical bonding, and its broader relevance in chemistry and beyond.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we pinpoint the number of valence electrons in sodium, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost energy level or shell of an atom. These electrons are the most loosely bound to the nucleus and are directly involved in chemical reactions and the formation of chemical bonds. The number of valence electrons an atom possesses determines its reactivity, the types of bonds it can form (ionic, covalent, metallic), and its overall chemical behavior. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often resembling the nearest noble gas. This drive toward stability is a cornerstone principle in chemistry, often referred to as the octet rule (although there are exceptions, particularly for elements beyond the second row of the periodic table).
Determining Sodium's Electronic Configuration
To determine the number of valence electrons in sodium, we need to examine its electronic configuration. The electronic configuration describes how electrons are distributed among the different energy levels and sublevels within an atom. Sodium's atomic number is 11, meaning it has 11 protons and 11 electrons in a neutral atom. Using the Aufbau principle and Hund's rule, we can systematically fill the electron orbitals:
- 1s²: The first energy level (n=1) can hold a maximum of two electrons, filling the 1s orbital.
- 2s²: The second energy level (n=2) begins with the 2s orbital, which also holds up to two electrons.
- 2p⁶: The second energy level continues with the 2p sublevel, which has three orbitals (px, py, pz), each capable of holding two electrons, for a total of six electrons.
- 3s¹: Finally, we reach the third energy level (n=3), beginning with the 3s orbital. Sodium's eleventh electron occupies this orbital.
Therefore, sodium's complete electronic configuration is 1s²2s²2p⁶3s¹.
Identifying Sodium's Valence Electrons
Now that we have the electronic configuration, identifying the valence electrons is straightforward. Valence electrons are those in the outermost shell, which, in sodium's case, is the third energy level (n=3). The third energy level contains only one electron, residing in the 3s orbital.
Therefore, sodium (Na) has only one valence electron.
Implications of Sodium's Single Valence Electron
The presence of just one valence electron profoundly influences sodium's chemical properties:
- Low Ionization Energy: Sodium readily loses its single valence electron to achieve a stable octet configuration, resembling neon (Ne). This ease of electron loss signifies a low ionization energy, making sodium highly reactive.
- Formation of Ionic Bonds: By losing its valence electron, sodium forms a +1 cation (Na⁺). This positively charged ion readily forms ionic bonds with electronegative elements, such as chlorine (Cl), resulting in the formation of ionic compounds like sodium chloride (NaCl), common table salt. The electrostatic attraction between the positively charged sodium ion and the negatively charged chloride ion holds the compound together.
- Metallic Bonding: In its elemental form, sodium exhibits metallic bonding. The valence electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged sodium ions. This electron sea facilitates electrical and thermal conductivity, typical characteristics of metals.
- Reactivity with Water: The single valence electron makes sodium highly reactive with water. The reaction is exothermic, producing hydrogen gas and sodium hydroxide, a strongly alkaline solution. This reaction is often demonstrated in chemistry classrooms but requires careful handling due to its vigorous nature.
Sodium's Role in Biology and Industry
Sodium's unique properties, stemming from its single valence electron, make it crucial in various biological and industrial applications:
Biological Significance:
- Nerve Impulse Transmission: Sodium ions play a critical role in transmitting nerve impulses throughout the body. The movement of sodium ions across cell membranes generates electrical signals that allow for communication between nerve cells and muscle cells.
- Fluid Balance: Sodium helps regulate the body's fluid balance, ensuring proper hydration and preventing dehydration.
- Nutrient Absorption: Sodium aids in the absorption of nutrients from the digestive system.
Industrial Applications:
- Production of Sodium Compounds: Sodium is a key reactant in the production of many commercially important compounds, including sodium hydroxide (NaOH), used in the manufacturing of soaps, detergents, and paper.
- Sodium Lamps: Sodium vapor lamps are known for their bright yellow light, used extensively in street lighting.
- Chemical Catalyst: Sodium compounds act as catalysts in various chemical reactions.
Beyond the Single Valence Electron: Exploring Related Concepts
While the single valence electron is a defining characteristic of sodium, understanding its properties requires considering other related concepts:
- Electron Affinity: Although sodium readily loses its valence electron, its electron affinity is relatively low. This means that it has a low tendency to gain an electron.
- Electronegativity: Sodium's electronegativity is low, indicating its low tendency to attract electrons in a chemical bond. This reinforces its tendency to lose its valence electron and form positive ions.
- Periodic Trends: Sodium's properties align with periodic trends observed across the alkali metals group (Group 1) in the periodic table. All alkali metals possess one valence electron and exhibit similar reactivity patterns.
Conclusion: A Single Electron with Profound Implications
In conclusion, sodium (Na) has one valence electron, a fact that profoundly shapes its chemical behavior, biological function, and industrial applications. Its readiness to lose this electron, resulting in a +1 cation, underlies its reactivity, its ability to form ionic bonds, and its role in numerous crucial processes. Understanding the significance of valence electrons, particularly in the case of sodium, is fundamental to comprehending the broader principles of chemical bonding, reactivity, and the behavior of matter. From the microscopic level of atomic interactions to the macroscopic world of industrial processes and biological systems, the impact of sodium's single valence electron is undeniable and far-reaching. Further exploration of these concepts will illuminate the intricate beauty and power of chemistry.
Latest Posts
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Na Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.