How Many Valence Electrons Does N Have
Holbox
Mar 15, 2025 · 6 min read
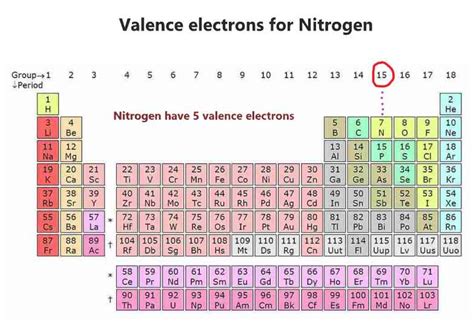
Table of Contents
How Many Valence Electrons Does Nitrogen (N) Have? A Deep Dive into Atomic Structure and Chemical Bonding
Determining the number of valence electrons an atom possesses is crucial for understanding its chemical behavior and how it forms bonds with other atoms. This article delves into the fascinating world of atomic structure, focusing specifically on nitrogen (N) and its valence electrons. We'll explore the underlying principles, explain how to determine valence electrons, and discuss the implications for nitrogen's reactivity and its role in various compounds.
Understanding Valence Electrons: The Key to Chemical Bonding
Valence electrons are the electrons located in the outermost shell, or energy level, of an atom. These electrons are the primary participants in chemical bonding, dictating how an atom interacts with other atoms to form molecules and compounds. The number of valence electrons determines an atom's bonding capacity – its ability to gain, lose, or share electrons to achieve a stable electron configuration.
Atoms strive for stability, typically represented by a full outermost electron shell. This stable configuration often mirrors the electron arrangement of noble gases, which are exceptionally unreactive due to their filled valence shells. This tendency to achieve stability drives chemical reactions and the formation of chemical bonds.
Determining the Number of Valence Electrons for Nitrogen (N)
Nitrogen, represented by the symbol N, is a nonmetal element located in Group 15 (also known as Group VA or the pnictogen group) of the periodic table. Its atomic number is 7, meaning it has 7 protons and 7 electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electron configuration.
Electron Configuration and Valence Shell
The electron configuration of nitrogen is 1s²2s²2p³. This notation describes how the electrons are distributed among the different energy levels and subshells within the atom.
- 1s²: Two electrons occupy the first energy level (n=1) in the s subshell.
- 2s²: Two electrons occupy the second energy level (n=2) in the s subshell.
- 2p³: Three electrons occupy the second energy level (n=2) in the p subshell.
The outermost shell of nitrogen is the second energy level (n=2), which contains both the 2s and 2p electrons. Therefore, nitrogen has a total of five valence electrons (2s²2p³). These five valence electrons are responsible for nitrogen's chemical behavior and its ability to form a variety of bonds.
The Role of the Periodic Table
The periodic table offers a convenient shortcut for determining the number of valence electrons. For main group elements (Groups 1-18), the group number (using the American system) directly indicates the number of valence electrons. Since nitrogen is in Group 15, it has 5 valence electrons. This is a useful rule of thumb, though it doesn't apply to transition metals.
Nitrogen's Chemical Behavior and Valence Electrons
The presence of five valence electrons profoundly influences nitrogen's chemical properties and its ability to form diverse chemical bonds. Nitrogen readily forms covalent bonds, sharing electrons with other atoms to achieve a stable octet (eight electrons in its outermost shell). This is a consequence of its relatively high electronegativity, meaning it has a strong tendency to attract electrons towards itself in a chemical bond.
Covalent Bonding in Nitrogen Compounds
Nitrogen's most common bonding pattern involves forming three covalent bonds. For example, in ammonia (NH₃), nitrogen shares three electrons with three hydrogen atoms, fulfilling the octet rule for nitrogen and providing each hydrogen with a stable duet (two electrons). In nitrogen gas (N₂), two nitrogen atoms form a triple bond, sharing three pairs of electrons to achieve octet stability for each nitrogen atom. This triple bond is incredibly strong, contributing to the inertness of nitrogen gas at room temperature.
Other Bonding Scenarios
While three covalent bonds are most common, nitrogen can sometimes participate in other bonding scenarios. For instance, in compounds like nitrogen oxides (NO, NO₂, N₂O₄, etc.), nitrogen can form various numbers of bonds, ranging from one to three, depending on the specific molecule and the oxidation state of the nitrogen atom. Understanding these diverse bonding patterns requires considering factors like formal charge, resonance structures, and molecular geometry.
Nitrogen's Importance in Biological Systems
Nitrogen is an essential element for life, playing a crucial role in the structure and function of many biological molecules. Its presence in amino acids, the building blocks of proteins, underscores its fundamental importance in biological systems. The nitrogen cycle, a complex series of processes that involve the transformation of nitrogen between various forms, is vital for the sustained productivity of ecosystems.
Nucleic Acids and Nitrogen
Nitrogen is also a critical component of nucleic acids, such as DNA and RNA, which carry the genetic information of all living organisms. The nitrogenous bases adenine, guanine, cytosine, and thymine (or uracil in RNA) all incorporate nitrogen atoms within their molecular structures, emphasizing the element's role in heredity and the transmission of genetic information across generations.
Nitrogen Fixation: A Biological Marvel
The conversion of atmospheric nitrogen gas (N₂) into ammonia (NH₃) – a process known as nitrogen fixation – is a crucial step in the nitrogen cycle. This process is carried out primarily by specialized microorganisms, allowing nitrogen to enter the biosphere and become available for plants and other organisms. The ability of certain bacteria to catalyze nitrogen fixation highlights the intricate interplay between biological systems and nitrogen's chemical properties.
Applications of Nitrogen and its Compounds
Nitrogen's versatility translates into a wide array of applications across numerous industries. Its inert nature makes it valuable as a protective atmosphere for food preservation and in various industrial processes. Ammonia, produced using the Haber-Bosch process, serves as a critical feedstock for fertilizers, dramatically impacting agricultural productivity worldwide. Other nitrogen-containing compounds find uses in pharmaceuticals, explosives, and various other materials.
Fertilizers and Agriculture
The pivotal role of nitrogen in plant growth has led to the widespread use of nitrogen-based fertilizers. These fertilizers supply plants with the necessary nitrogen for protein synthesis and other metabolic processes, enhancing crop yields and contributing to food security. However, excessive use of nitrogen fertilizers can lead to environmental issues such as water pollution and greenhouse gas emissions, highlighting the need for responsible and sustainable agricultural practices.
Industrial Applications
Beyond agriculture, nitrogen finds numerous applications in diverse industries. Its inertness allows its use as a protective atmosphere in packaging to prevent oxidation and spoilage of food products and other materials. Nitrogen gas is also employed in various industrial processes, such as cooling systems and as a propellant in aerosol cans. Various nitrogen compounds find use in the production of plastics, fibers, and other materials, further highlighting its significant industrial importance.
Conclusion: Nitrogen's Valence Electrons and Their Significance
Nitrogen, with its five valence electrons, displays a rich and varied chemistry, participating in covalent bonds to achieve stability. Its capacity to form diverse compounds, ranging from simple molecules like ammonia to complex biological molecules like proteins and nucleic acids, underscores the profound impact of its electronic structure. Understanding the number of valence electrons in nitrogen is fundamental to comprehending its reactivity, its role in biological systems, and its widespread applications in various industries. Further exploration into nitrogen chemistry reveals fascinating insights into the intricate relationships between atomic structure, chemical behavior, and the material world around us. The study of valence electrons is essential for gaining a deep appreciation of the fundamental principles governing chemical bonding and the vast diversity of chemical compounds found in nature and created through human ingenuity.
Latest Posts
Latest Posts
-
Select The Correct Definition Of The Term Comparative Advantage
Mar 15, 2025
-
A Pharmaceutical Company Receives Large Shipments Of Aspirin Tablets
Mar 15, 2025
-
Many Strategists Argue That Firms Should Centralize R
Mar 15, 2025
-
The Functioning Of Enhancers Is An Example Of
Mar 15, 2025
-
A Company Exhibits Responsible Corporate Citizenship When It
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does N Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
