How Many Different Molecules Are Drawn Below
Holbox
Mar 26, 2025 · 5 min read
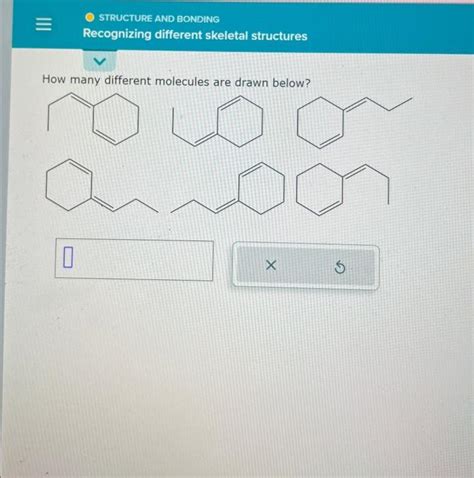
Table of Contents
- How Many Different Molecules Are Drawn Below
- Table of Contents
- Deconstructing Molecular Diversity: How Many Different Molecules Are Drawn Below?
- The Fundamentals: Types of Isomerism
- 1. Constitutional Isomers (Structural Isomers):
- 2. Stereoisomers:
- A Systematic Approach to Counting Unique Molecules
- Practical Applications and Beyond
- Conclusion: Mastering Molecular Identification
- Latest Posts
- Latest Posts
- Related Post
Deconstructing Molecular Diversity: How Many Different Molecules Are Drawn Below?
This article delves into the fascinating world of molecular structures and tackles the crucial question of identifying unique molecules within a given set of drawings. While the "drawings below" are absent in your prompt, we will explore the principles and techniques used to determine the number of distinct molecules represented, focusing on isomerism, chirality, and other key concepts. This will provide a framework you can apply to any set of molecular drawings you may encounter.
This comprehensive guide will equip you with the knowledge to approach such problems systematically, offering a detailed explanation of the underlying chemical principles and strategies for identifying unique molecular entities. We'll cover topics like:
- Understanding Isomerism: The various types of isomers (structural, geometric, stereoisomers) and how they influence molecular identity.
- Chirality and Enantiomers: The importance of chiral centers and the distinction between enantiomers and diastereomers.
- Constitutional Isomers: Identifying variations in connectivity of atoms within the molecule.
- Stereoisomers: Distinguishing molecules with the same connectivity but different spatial arrangements.
- Systematic Approaches to Counting Unique Molecules: Step-by-step methods for analyzing complex sets of molecular drawings.
- Applying this knowledge to real-world scenarios: Illustrating the practical applications of molecular identification in fields such as drug discovery and material science.
The Fundamentals: Types of Isomerism
Before we tackle counting molecules, understanding isomerism is paramount. Isomers are molecules that share the same molecular formula but differ in their arrangement of atoms. There are several key types:
1. Constitutional Isomers (Structural Isomers):
These isomers differ in the connectivity of their atoms. This means the atoms are bonded together in a different order. For example, consider C₄H₁₀. There are two constitutional isomers: butane (a straight chain) and methylpropane (a branched chain). They have the same molecular formula but different structural arrangements.
Example:
Imagine two molecules with the formula C₃H₈O. One could be propan-1-ol (where the hydroxyl group, -OH, is attached to a terminal carbon), and the other could be propan-2-ol (where the -OH group is attached to the central carbon). These are constitutional isomers because the connectivity of the atoms is different.
2. Stereoisomers:
Stereoisomers have the same molecular formula and the same connectivity of atoms but differ in the three-dimensional arrangement of their atoms in space. This category encompasses several subtypes:
a) Geometric Isomers (cis-trans isomers):
These arise from restricted rotation around a double bond or a ring. The atoms or groups are arranged differently on either side of the restricted bond. A classic example is cis- and trans-but-2-ene.
Example:
Consider a molecule with a carbon-carbon double bond and two different groups attached to each carbon. The cis isomer has the same groups on the same side of the double bond, while the trans isomer has them on opposite sides.
b) Enantiomers (Optical Isomers):
These are non-superimposable mirror images of each other. They possess at least one chiral center (a carbon atom with four different groups attached). Enantiomers have identical physical properties except for their interaction with plane-polarized light (they rotate the plane of polarized light in opposite directions).
Example:
Consider the molecule lactic acid. It has one chiral center. The two enantiomers are designated as (R)-lactic acid and (S)-lactic acid based on the Cahn-Ingold-Prelog priority rules.
c) Diastereomers:
These are stereoisomers that are not mirror images of each other. They can have multiple chiral centers, and the spatial arrangement of groups around these centers differs. Diastereomers have different physical and chemical properties.
Example:
Consider a molecule with two chiral centers. There are four possible stereoisomers. The pair of enantiomers will be mirror images, but other pairs within the four stereoisomers are diastereomers, as they are not mirror images and possess different physical and chemical properties.
A Systematic Approach to Counting Unique Molecules
Let's outline a step-by-step approach for determining the number of unique molecules from a set of drawings:
-
Identify the Molecular Formula: Determine the number and type of atoms present in each molecule.
-
Categorize Isomers: Identify constitutional isomers (differences in connectivity) and stereoisomers (differences in spatial arrangement).
-
Analyze Constitutional Isomers: Carefully examine the connectivity of each molecule. Ensure you don't count the same connectivity twice, even if they are drawn differently.
-
Analyze Stereoisomers: For each constitutional isomer, identify the presence of chiral centers and double bonds. Determine the number of possible stereoisomers (enantiomers and diastereomers) for each.
-
Eliminate Duplicates: Ensure that you are not counting any molecule more than once. Identical molecules drawn differently are still the same molecule.
-
Sum Unique Molecules: After carefully categorizing and eliminating duplicates, add up the total number of unique molecules across all types of isomerism.
Practical Applications and Beyond
The ability to accurately identify and distinguish molecules is crucial in various scientific disciplines. In drug discovery, identifying different isomers is essential as they can have vastly different biological activities. Some isomers may be therapeutically active, while others might be inactive or even toxic. Similarly, in material science, the arrangement of atoms can significantly impact the material's properties, making the ability to differentiate between molecules critical for designing materials with specific characteristics.
Conclusion: Mastering Molecular Identification
Identifying the number of unique molecules presented in a set of drawings involves a systematic approach that integrates knowledge of various types of isomerism, including constitutional and stereoisomers (geometric, enantiomers, and diastereomers). By systematically analyzing the connectivity and spatial arrangements of atoms, you can effectively identify and count unique molecular entities. This skill is crucial in diverse fields, showcasing the significant practical implications of understanding molecular structure and isomerism. Remember, practice is key! Work through numerous examples to hone your skills and confidently distinguish between subtly different molecules. The more you practice, the easier it will become to identify unique molecular structures and apply this knowledge to solve a range of chemical challenges.
Latest Posts
Latest Posts
-
Formal Education As An Approach To Employee Development Includes
Mar 29, 2025
-
Vail Company Recorded The Following Transactions During November
Mar 29, 2025
-
What Coversheet Is Attached To Help Protect A Secret Document
Mar 29, 2025
-
Elimination Reactions Are Favored Over Nucleophilic Substitution Reactions
Mar 29, 2025
-
One Important Role Of Purchasing Is To
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Different Molecules Are Drawn Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
