Give The Major Product Of The Following Reaction
Holbox
Mar 10, 2025 · 6 min read
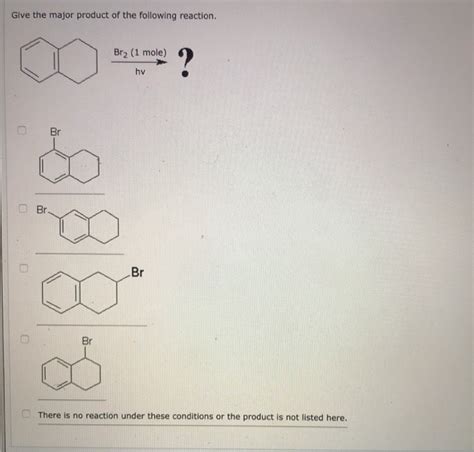
Table of Contents
Predicting the Major Product of Organic Reactions: A Comprehensive Guide
Predicting the major product of an organic reaction is a crucial skill for any aspiring chemist. It requires a deep understanding of reaction mechanisms, functional group transformations, and the interplay of various factors that influence reaction pathways. This article explores the major product prediction for several common reaction types, providing a detailed explanation of the underlying principles. We'll delve into reaction mechanisms, stereochemistry, and regiochemistry to enhance your understanding and prediction accuracy. This comprehensive guide will equip you with the tools necessary to tackle a wide array of organic reactions with confidence.
Understanding Reaction Mechanisms: The Key to Prediction
Before we jump into specific reactions, let's establish the importance of understanding reaction mechanisms. A reaction mechanism is a step-by-step description of how reactants transform into products. It reveals the sequence of bond breaking and bond formation events, involving intermediates and transition states. By understanding the mechanism, we can identify the most likely pathway and thus predict the major product. Different factors influence the mechanism, including:
-
Nature of the reactants: The functional groups present and their electronic properties significantly influence reactivity. For example, electron-rich nucleophiles will react differently than electron-deficient electrophiles.
-
Reaction conditions: Temperature, solvent, and the presence of catalysts or reagents can dramatically alter the reaction pathway. Acidic or basic conditions can favor different mechanisms.
-
Steric hindrance: The size and shape of molecules can affect their reactivity. Bulky groups can hinder the approach of reagents, leading to different products.
Key Reaction Types and Major Product Prediction
Let's explore several common reaction types and the principles behind predicting their major products.
1. SN1 and SN2 Reactions: Nucleophilic Substitution
These reactions involve the substitution of a leaving group by a nucleophile. They differ significantly in their mechanisms and, therefore, their stereochemical outcomes.
-
SN2 Reactions: These are concerted reactions, meaning the bond breaking and bond formation occur simultaneously. They proceed through a single transition state. SN2 reactions are stereospecific, leading to inversion of configuration at the chiral center. Strong nucleophiles and primary or secondary alkyl halides favor SN2 reactions. The major product will be the result of nucleophilic attack on the backside of the carbon bearing the leaving group.
-
SN1 Reactions: These are two-step reactions. The first step involves the formation of a carbocation intermediate, which is then attacked by the nucleophile. SN1 reactions are not stereospecific, leading to racemization if the carbocation is not stabilized. Tertiary alkyl halides and weak nucleophiles favor SN1 reactions. The major product will be determined by the stability of the carbocation intermediate; more substituted carbocations are more stable (tertiary > secondary > primary). Rearrangements are possible in SN1 reactions if a more stable carbocation can be formed via hydride or alkyl shifts.
2. E1 and E2 Reactions: Elimination Reactions
Elimination reactions involve the removal of a leaving group and a proton from adjacent carbon atoms, leading to the formation of a double bond (alkene).
-
E2 Reactions: These are concerted reactions, occurring in a single step. A strong base abstracts a proton while simultaneously the leaving group departs. E2 reactions are stereospecific, favoring anti-periplanar geometry (leaving group and proton on opposite sides of the molecule). The major product is usually the more substituted alkene (Zaitsev's rule), due to increased stability.
-
E1 Reactions: These are two-step reactions. The first step involves the formation of a carbocation intermediate, followed by proton abstraction. E1 reactions are not stereospecific. The major product will be the more substituted alkene (Zaitsev's rule) due to the greater stability of the carbocation intermediate. Rearrangements are possible in E1 reactions.
3. Electrophilic Aromatic Substitution
These reactions involve the substitution of a hydrogen atom on an aromatic ring by an electrophile. The electrophile attacks the aromatic ring, forming a carbocation intermediate (arenium ion), which then loses a proton to restore aromaticity. The major product is influenced by the directing effects of substituents already present on the ring.
-
Ortho/Para directing groups: These groups donate electrons to the ring, activating it towards electrophilic attack and directing the electrophile to the ortho and para positions. Examples include -OH, -NH2, -OCH3.
-
Meta directing groups: These groups withdraw electrons from the ring, deactivating it towards electrophilic attack and directing the electrophile to the meta position. Examples include -NO2, -COOH, -SO3H.
4. Addition Reactions: Alkenes and Alkynes
Alkenes and alkynes undergo addition reactions, where atoms or groups are added across the double or triple bond. The mechanism and regiochemistry (position of addition) depend on the nature of the reagent.
-
Electrophilic addition: This occurs when an electrophile attacks the double or triple bond, forming a carbocation intermediate. Markovnikov's rule predicts the major product: the electrophile adds to the carbon atom with more alkyl substituents (more substituted carbon).
-
Nucleophilic addition: This occurs when a nucleophile attacks the double or triple bond. Anti-Markovnikov addition can occur in the presence of peroxides (radical mechanism).
5. Grignard Reactions
Grignard reagents (RMgX) are powerful nucleophiles that react with carbonyl compounds (aldehydes, ketones, esters, and carboxylic acids). The major product depends on the type of carbonyl compound.
-
Reaction with aldehydes: Leads to secondary alcohols.
-
Reaction with ketones: Leads to tertiary alcohols.
-
Reaction with esters: Leads to tertiary alcohols.
-
Reaction with carboxylic acids: Leads to carboxylic acid salts.
Factors Affecting Product Distribution
Beyond the basic mechanisms, several factors can influence the major product:
-
Steric effects: Bulky substituents can hinder reagent access, leading to the formation of less sterically hindered products.
-
Electronic effects: Electron-donating or withdrawing groups can influence the reactivity of different sites in a molecule.
-
Kinetic vs. thermodynamic control: Some reactions can lead to different products depending on whether the reaction is under kinetic or thermodynamic control. Kinetic control favors the faster reaction, while thermodynamic control favors the more stable product.
-
Solvent effects: The solvent can influence the reaction rate and selectivity. Polar solvents can stabilize charged intermediates, affecting reaction pathways.
Advanced Concepts: Stereochemistry and Regiochemistry
Understanding stereochemistry and regiochemistry is crucial for accurate product prediction.
-
Stereochemistry: Deals with the three-dimensional arrangement of atoms in a molecule. Reactions can be stereospecific (product stereochemistry is determined by reactant stereochemistry) or stereoselective (one stereoisomer is formed preferentially).
-
Regiochemistry: Deals with the orientation of addition or substitution in a molecule. Markovnikov's rule is a key concept in predicting regiochemistry in electrophilic addition reactions.
Practical Application and Problem Solving
Predicting the major product of a reaction involves a systematic approach:
-
Identify the functional groups: Determine the reactants and their functional groups.
-
Determine the reaction type: Classify the reaction (SN1, SN2, E1, E2, addition, etc.).
-
Consider the reaction mechanism: Understand the step-by-step process.
-
Identify the major intermediate: Determine the most stable intermediate.
-
Predict the product: Based on the mechanism and the stability of intermediates, predict the major product.
-
Consider stereochemistry and regiochemistry: Determine the stereochemistry and regiochemistry of the product.
-
Consider side reactions: Account for potential side reactions that might compete with the main reaction.
Conclusion: Mastering Product Prediction
Predicting the major product of organic reactions is a challenging but rewarding skill. By understanding reaction mechanisms, stereochemistry, regiochemistry, and the various factors that influence reaction pathways, you can significantly improve your ability to accurately predict the outcome of organic reactions. This comprehensive guide provides a solid foundation for tackling a wide array of organic chemistry problems. Consistent practice and a deep understanding of the underlying principles are key to mastering this essential skill. Remember to systematically analyze each reaction, considering all relevant factors to arrive at an accurate prediction. With diligent effort, you'll enhance your proficiency in organic chemistry and confidently predict the major products of diverse reactions.
Latest Posts
Related Post
Thank you for visiting our website which covers about Give The Major Product Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
