Give The Iupac Names Of The Following Compounds
Holbox
Mar 30, 2025 · 7 min read
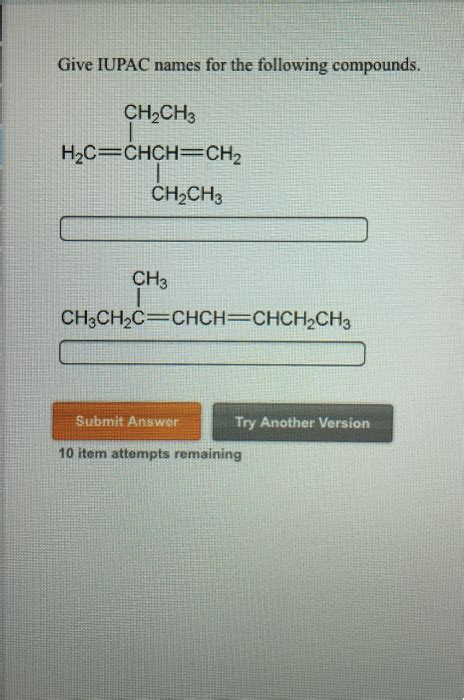
Table of Contents
- Give The Iupac Names Of The Following Compounds
- Table of Contents
- Mastering IUPAC Nomenclature: A Comprehensive Guide with Examples
- Understanding the Fundamentals of IUPAC Nomenclature
- Alkanes: The Foundation of Organic Chemistry
- Branched Alkanes: Introducing Substituents
- Alkenes and Alkynes: Incorporating Double and Triple Bonds
- Cycloalkanes and Cycloalkenes: Ring Structures
- Alcohols, Ethers, and Halides: Functional Groups
- Aldehydes and Ketones: Carbonyl Compounds
- Carboxylic Acids, Esters, Amides, and Nitriles: Other Important Functional Groups
- Polyfunctional Compounds: Molecules with Multiple Functional Groups
- Latest Posts
- Latest Posts
- Related Post
Mastering IUPAC Nomenclature: A Comprehensive Guide with Examples
Naming organic compounds might seem daunting at first, but with a systematic approach, it becomes a manageable and even enjoyable skill. The International Union of Pure and Applied Chemistry (IUPAC) provides a standardized system for naming organic compounds, ensuring clear and unambiguous communication among chemists worldwide. This comprehensive guide will delve into the intricacies of IUPAC nomenclature, providing you with the tools to confidently name a wide variety of organic molecules. We will explore various functional groups, parent chains, prefixes, suffixes, and numbering systems, all while illustrating the principles with numerous examples.
Understanding the Fundamentals of IUPAC Nomenclature
Before diving into complex examples, let's establish a solid foundation. IUPAC nomenclature relies on a set of rules that prioritize clarity and consistency. The core principles include:
-
Identifying the Parent Chain: This is the longest continuous carbon chain in the molecule. If multiple chains of equal length exist, the chain with the most substituents is chosen.
-
Numbering the Carbon Atoms: The parent chain is numbered to provide the lowest possible numbers for substituents. Numbering starts from the end closest to the first substituent encountered.
-
Identifying Substituents: These are atoms or groups of atoms attached to the parent chain. They are named systematically and their positions indicated by numbers.
-
Alphabetical Ordering of Substituents: Substituents are listed alphabetically in the name, ignoring prefixes like di, tri, etc., except for prefixes denoting position like iso, tert, sec. However, these prefixes are considered when alphabetizing.
-
Using Prefixes to Indicate Multiple Substituents: Prefixes like di, tri, tetra, etc., are used to indicate the presence of multiple identical substituents.
-
Using Hyphens and Commas: Hyphens are used to separate numbers from words, and commas separate numbers from each other.
Alkanes: The Foundation of Organic Chemistry
Alkanes, the simplest organic compounds, consist solely of carbon and hydrogen atoms linked by single bonds. Their IUPAC names follow a straightforward pattern:
- Meth- (1 carbon): Methane (CH₄)
- Eth- (2 carbons): Ethane (C₂H₆)
- Prop- (3 carbons): Propane (C₃H₈)
- But- (4 carbons): Butane (C₄H₁₀)
- Pent- (5 carbons): Pentane (C₅H₁₂)
- Hex- (6 carbons): Hexane (C₆H₁₄)
- Hept- (7 carbons): Heptane (C₇H₁₆)
- Oct- (8 carbons): Octane (C₈H₁₈)
- Non- (9 carbons): Nonane (C₉H₂₀)
- Dec- (10 carbons): Decane (C₁₀H₂₂)
For alkanes with more than 10 carbons, Greek numerical prefixes are used (e.g., undecane, dodecane, etc.).
Branched Alkanes: Introducing Substituents
Branched alkanes have alkyl groups attached to the parent chain. Alkyl groups are derived from alkanes by removing one hydrogen atom. Common alkyl groups include:
- Methyl (CH₃): Derived from methane
- Ethyl (CH₂CH₃): Derived from ethane
- Propyl (CH₂CH₂CH₃): Derived from propane
- Isopropyl (CH(CH₃)₂): A branched propyl group
- Butyl (C₄H₉): Derived from butane (has several isomers)
Example 1:
Consider the branched alkane with the structure: CH₃CH(CH₃)CH₂CH₃
-
Identify the parent chain: The longest continuous chain contains four carbons, making it a butane derivative.
-
Number the carbons: Numbering from the left gives the methyl substituent a lower number (2) than numbering from the right (3).
-
Name the substituent: The substituent is a methyl group.
-
Combine the information: The IUPAC name is 2-methylbutane.
Example 2: A more complex example: CH₃CH₂CH(CH₃)CH(CH₂CH₃)CH₃
-
Parent Chain: The longest chain has six carbons (hexane).
-
Numbering: Numbering from the left prioritizes lower numbers for the substituents (2-methyl, 4-ethyl).
-
Substituents: A methyl group on carbon 2 and an ethyl group on carbon 4.
-
Name: 4-ethyl-2-methylhexane (Note the alphabetical ordering of ethyl before methyl).
Alkenes and Alkynes: Incorporating Double and Triple Bonds
Alkenes contain at least one carbon-carbon double bond, and alkynes contain at least one carbon-carbon triple bond. The nomenclature follows similar principles:
-
The parent chain must include the double or triple bond.
-
Numbering prioritizes the lowest number for the double or triple bond.
-
The suffix "-ene" is used for alkenes, and "-yne" is used for alkynes.
-
The position of the double or triple bond is indicated by a number before the suffix.
Example 3: CH₂=CHCH₂CH₃
This is an alkene with four carbons (butene). The double bond starts at carbon 1. The name is 1-butene.
Example 4: CH₃C≡CCH₃
This is an alkyne with four carbons (butyne). The triple bond is between carbons 2 and 3. The name is 2-butyne.
Cycloalkanes and Cycloalkenes: Ring Structures
Cycloalkanes are saturated cyclic hydrocarbons, and cycloalkenes contain at least one carbon-carbon double bond within the ring. Their names are prefixed with "cyclo-":
Example 5: A five-membered ring of carbons: cyclopentane.
Example 6: A six-membered ring with a double bond between carbons 1 and 2: 1-cyclohexene. (Note that numbering starts at a carbon in the double bond and proceeds in the direction that gives the next substituent the lowest number.)
Alcohols, Ethers, and Halides: Functional Groups
Functional groups significantly influence the properties and reactivity of organic compounds. Their presence is indicated by specific suffixes or prefixes in IUPAC names:
-
Alcohols (-OH): The suffix "-ol" is added to the alkane name. The position of the hydroxyl group (-OH) is indicated by a number. For example, CH₃CH₂OH is ethanol.
-
Ethers (R-O-R'): Ethers are named by listing the alkyl groups alphabetically followed by the word "ether". For example, CH₃OCH₂CH₃ is ethyl methyl ether.
-
Haloalkanes (F, Cl, Br, I): Halogen substituents (fluoro-, chloro-, bromo-, iodo-) are treated as prefixes and are listed alphabetically. For example, CH₃CHClCH₃ is 2-chloropropane.
Example 7: CH₃CH(OH)CH₂CH₃ is 2-butanol.
Example 8: CH₃CH₂OCH₃ is methyl ethyl ether.
Example 9: CH₂ClCH₂CH₃ is 1-chloropropane.
Aldehydes and Ketones: Carbonyl Compounds
Aldehydes and ketones contain the carbonyl group (C=O).
-
Aldehydes: The suffix "-al" is added to the alkane name. The carbonyl group is always at the end of the chain, so no number is needed. For example, CH₃CHO is ethanal.
-
Ketones: The suffix "-one" is used. The position of the carbonyl group is indicated by a number. For example, CH₃COCH₃ is propan-2-one (commonly called acetone).
Example 10: CH₃(CH₂)₄CHO is hexanal.
Example 11: CH₃CH₂COCH₃ is butan-2-one.
Carboxylic Acids, Esters, Amides, and Nitriles: Other Important Functional Groups
-
Carboxylic Acids (-COOH): The suffix "-oic acid" is used. For example, CH₃COOH is ethanoic acid (acetic acid).
-
Esters (RCOOR'): Esters are named by first naming the alkyl group (R') attached to the oxygen, followed by the name of the carboxylate group (RCOO-), with the suffix "-oate" replacing "-oic acid". For example, CH₃COOCH₂CH₃ is ethyl ethanoate (ethyl acetate).
-
Amides (RCONH₂): The suffix "-amide" is used. For example, CH₃CONH₂ is ethanamide.
-
Nitriles (-CN): The suffix "-nitrile" is used. For example, CH₃CN is ethanenitrile.
Example 12: CH₃CH₂CH₂COOH is butanoic acid.
Example 13: CH₃COOCH₃ is methyl ethanoate.
Example 14: CH₃CH₂CONH₂ is propanamide.
Example 15: CH₃CH₂CN is propanenitrile.
Polyfunctional Compounds: Molecules with Multiple Functional Groups
When a molecule contains multiple functional groups, the order of priority in naming is crucial. The highest-priority group determines the suffix, while other groups are treated as prefixes. The priority order generally follows:
- Carboxylic acids
- Anhydrides
- Esters
- Amides
- Nitriles
- Aldehydes
- Ketones
- Alcohols
- Amines
- Alkenes
- Alkynes
- Alkanes
Example 16: A molecule containing both a carboxylic acid and an alcohol group. The carboxylic acid gets priority, giving the suffix "-oic acid." The alcohol is treated as a prefix "-hydroxy-".
This detailed guide provides a solid foundation in IUPAC nomenclature. Remember that practice is key to mastering this skill. Work through numerous examples, focusing on identifying the parent chain, numbering correctly, and properly ordering substituents. The more you practice, the more confident and proficient you'll become in naming and understanding the structures of organic compounds.
Latest Posts
Latest Posts
-
Percent Of Oxygen In Potassium Chlorate Lab Answers
Apr 02, 2025
-
A Local School Administrator Observes An Increase
Apr 02, 2025
-
Noticing That You Have Difficulty Concentrating
Apr 02, 2025
-
Which Of The Following Correctly Explains The Actions An Agent
Apr 02, 2025
-
What Is The Common Name Of The Following Compound
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Give The Iupac Names Of The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
