For The Sn1 Reaction Draw The Major Organic Product
Holbox
Apr 05, 2025 · 6 min read
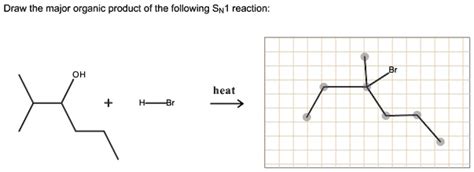
Table of Contents
- For The Sn1 Reaction Draw The Major Organic Product
- Table of Contents
- For the SN1 Reaction: Drawing the Major Organic Product – A Comprehensive Guide
- Understanding the SN1 Mechanism
- Step 1: Ionization
- Step 2: Nucleophilic Attack
- Factors Affecting the SN1 Reaction
- 1. Substrate Structure
- 2. Leaving Group Ability
- 3. Nucleophile Strength
- 4. Solvent
- Predicting the Major Organic Product
- Carbocation Rearrangements: A Complication
- Examples
- Practice Problems
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
For the SN1 Reaction: Drawing the Major Organic Product – A Comprehensive Guide
The SN1 reaction, or unimolecular nucleophilic substitution, is a fundamental concept in organic chemistry. Understanding how to predict the major organic product formed in an SN1 reaction is crucial for any aspiring chemist. This comprehensive guide will delve into the intricacies of SN1 reactions, providing a step-by-step approach to identifying the major product and highlighting key factors influencing the reaction outcome.
Understanding the SN1 Mechanism
Before we dive into predicting the major product, let's recap the SN1 mechanism. This reaction proceeds through a two-step process:
Step 1: Ionization
The first step involves the leaving group departing from the substrate, forming a carbocation intermediate. This step is rate-determining, meaning its speed dictates the overall reaction rate. The stability of the carbocation significantly influences the rate. Tertiary (3°) carbocations are the most stable, followed by secondary (2°), and primary (1°) carbocations are the least stable. Methyl carbocations are extremely unstable.
Step 2: Nucleophilic Attack
In the second step, the nucleophile (Nu⁻) attacks the carbocation, forming a new bond and generating the product. Because the carbocation is planar, the nucleophile can attack from either side, leading to the possibility of stereoisomers.
Factors Affecting the SN1 Reaction
Several factors can influence the outcome of an SN1 reaction and the formation of the major product:
1. Substrate Structure
The stability of the carbocation intermediate is paramount. A more stable carbocation will form more readily, leading to a faster reaction. The order of carbocation stability is: 3° > 2° > 1° > methyl. Therefore, tertiary substrates overwhelmingly favor SN1 reactions.
2. Leaving Group Ability
A good leaving group is essential for an SN1 reaction. Good leaving groups are typically weak bases that can stabilize the negative charge after departing. Examples include I⁻, Br⁻, Cl⁻, and tosylate (OTs). The better the leaving group, the faster the reaction.
3. Nucleophile Strength
While the nucleophile's strength isn't as crucial as in SN2 reactions, it still plays a role. Weak nucleophiles are generally preferred for SN1 reactions to avoid competing SN2 pathways. Strong nucleophiles often lead to a mixture of SN1 and SN2 products.
4. Solvent
The solvent plays a critical role in stabilizing the carbocation intermediate. Polar protic solvents, such as water, alcohols, and carboxylic acids, are ideal for SN1 reactions because they can solvate both the carbocation and the leaving group, reducing the energy barrier for ionization.
Predicting the Major Organic Product
Now, let's apply this knowledge to predict the major organic product in an SN1 reaction. Consider the following steps:
-
Identify the Substrate: Determine the structure of the alkyl halide or other substrate undergoing the reaction. Note the degree of substitution (1°, 2°, 3°).
-
Identify the Leaving Group: Locate the leaving group within the substrate. Assess its ability to leave as a stable anion.
-
Identify the Nucleophile: Determine the nucleophile participating in the reaction.
-
Determine the Carbocation Intermediate: Imagine the leaving group departing, creating a carbocation. Evaluate the stability of this carbocation. Remember, tertiary carbocations are the most stable, followed by secondary, and then primary. If rearrangements are possible (discussed below), consider them.
-
Nucleophilic Attack: The nucleophile will attack the carbocation. Since the carbocation is planar, attack can occur from either side, leading to a racemic mixture (equal amounts of both enantiomers) if the starting material is chiral.
-
Draw the Product: Draw the structure of the product formed after the nucleophile attacks the carbocation.
Carbocation Rearrangements: A Complication
Carbocation rearrangements can significantly impact the major product formed in SN1 reactions. These rearrangements involve the shift of a hydride (H⁻) or an alkyl group to a more stable carbocation position. This often results in a more substituted, and thus more stable, carbocation. Hydride shifts and alkyl shifts are common rearrangement types.
-
Hydride shift: A hydrogen atom with its bonding electrons migrates from an adjacent carbon to the carbocation center.
-
Alkyl shift: An alkyl group with its bonding electrons migrates from an adjacent carbon to the carbocation center.
The rearrangement will occur if it leads to a more stable carbocation (e.g., converting a secondary carbocation to a tertiary carbocation). This changes the structure of the final product.
Examples
Let's illustrate this with some examples:
Example 1: SN1 reaction of tert-butyl bromide with water
- Substrate: tert-butyl bromide (3° alkyl halide)
- Leaving group: Br⁻ (good leaving group)
- Nucleophile: H₂O (weak nucleophile)
- Carbocation: A tertiary carbocation is formed, which is relatively stable. No rearrangement is possible.
- Nucleophilic Attack: Water attacks the carbocation.
- Product: tert-butyl alcohol is the major product. A racemic mixture is formed due to attack from either side of the planar carbocation.
Example 2: SN1 reaction of 3-bromo-3-methylpentane with methanol
- Substrate: 3-bromo-3-methylpentane (3° alkyl halide)
- Leaving group: Br⁻
- Nucleophile: CH₃OH (weak nucleophile)
- Carbocation: A tertiary carbocation forms. No rearrangement is necessary as it is already tertiary.
- Nucleophilic Attack: Methanol attacks the carbocation.
- Product: 3-methoxy-3-methylpentane is the major product, again a racemic mixture.
Example 3: SN1 reaction of 2-bromo-3-methylbutane with ethanol
- Substrate: 2-bromo-3-methylbutane (secondary alkyl halide)
- Leaving group: Br⁻
- Nucleophile: CH₃CH₂OH
- Carbocation: A secondary carbocation forms initially. However, a hydride shift is possible, leading to a more stable tertiary carbocation. The hydride shift from the methyl group to the carbocation center generates a tertiary carbocation.
- Nucleophilic Attack: Ethanol attacks the rearranged tertiary carbocation.
- Product: The major product will be the ether derived from the tertiary carbocation after ethanol attacks. Again a racemic mixture.
Practice Problems
To solidify your understanding, try predicting the major organic products for the following SN1 reactions:
- 2-chloro-2-methylpropane + H₂O
- 2-bromo-2-methylbutane + CH₃CH₂OH
- 1-bromo-2-methylpropane + CH₃COOH
Remember to consider the stability of the carbocation intermediate and the possibility of rearrangements. This will help you accurately predict the major product formed in each SN1 reaction.
Conclusion
Predicting the major organic product in an SN1 reaction requires a thorough understanding of the mechanism, the factors influencing the reaction, and the possibility of carbocation rearrangements. By systematically analyzing the substrate, leaving group, nucleophile, and solvent, you can confidently determine the structure and stereochemistry of the major product. Practice is key to mastering this skill, so work through numerous examples to build your proficiency. This comprehensive guide provides a robust foundation for understanding and predicting the outcome of SN1 reactions.
Latest Posts
Latest Posts
-
How Is Alveolar Air Different Than Inspired Air
Apr 11, 2025
-
Operant Conditioning Most Importantly Involves Forming Associations Between
Apr 11, 2025
-
Drag The Line To Finish The Sequence
Apr 11, 2025
-
Which Criterion Fo Rmarket Segmentation Is Lest Liekto To Enable
Apr 11, 2025
-
A Special Order Generally Should Be Accepted If
Apr 11, 2025
Related Post
Thank you for visiting our website which covers about For The Sn1 Reaction Draw The Major Organic Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.