For Each Solute Click The Button Under The Better Solvent
Holbox
Mar 23, 2025 · 6 min read
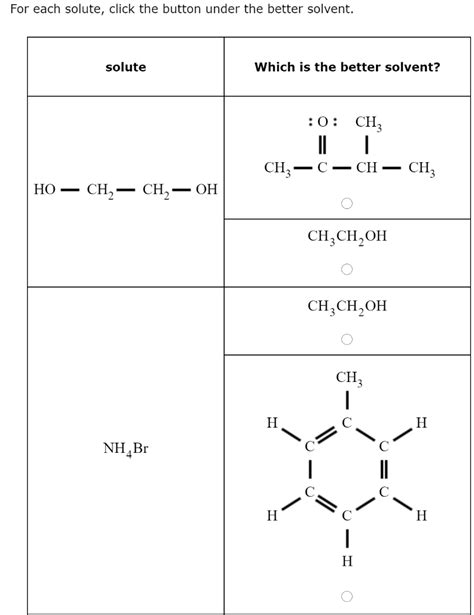
Table of Contents
- For Each Solute Click The Button Under The Better Solvent
- Table of Contents
- Choosing the Right Solvent: A Comprehensive Guide for Each Solute
- Understanding the "Like Dissolves Like" Principle
- Examples of Polar Solutes and Solvents:
- Examples of Nonpolar Solutes and Solvents:
- Factors Influencing Solvent Choice Beyond "Like Dissolves Like"
- 1. Solvent Polarity: A Deeper Dive
- 2. Hydrogen Bonding Capacity
- 3. Solvent Viscosity
- 4. Temperature
- 5. Pressure
- 6. Solvent Toxicity and Safety
- Practical Guide to Solvent Selection for Different Solutes
- Conclusion: A Holistic Approach to Solvent Selection
- Latest Posts
- Latest Posts
- Related Post
Choosing the Right Solvent: A Comprehensive Guide for Each Solute
Selecting the appropriate solvent for a given solute is a fundamental aspect of chemistry and numerous other scientific disciplines. The choice hinges on several key factors, primarily the solubility of the solute in the solvent, which is governed by the principle "like dissolves like." This principle dictates that polar solvents effectively dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes. However, the reality is often more nuanced, demanding a deeper understanding of intermolecular forces and solvent properties. This article provides a detailed exploration of solvent selection, explaining the underlying principles and offering a practical guide for choosing the best solvent for various solutes.
Understanding the "Like Dissolves Like" Principle
The fundamental rule governing solute-solvent interactions is the principle of "like dissolves like." This simple statement encapsulates a complex interplay of intermolecular forces. Polar solutes, possessing a significant dipole moment due to uneven electron distribution, readily dissolve in polar solvents. These solvents also possess significant dipole moments, enabling strong dipole-dipole interactions or even hydrogen bonding with the solute molecules.
Examples of Polar Solutes and Solvents:
-
Solute: Sugar (sucrose), a polar molecule due to numerous hydroxyl (-OH) groups.
-
Solvent: Water (H₂O), a highly polar solvent with strong hydrogen bonding capabilities. The hydroxyl groups on sugar readily form hydrogen bonds with water molecules, leading to high solubility.
-
Solute: Sodium chloride (NaCl), an ionic compound with strong electrostatic interactions between Na⁺ and Cl⁻ ions.
-
Solvent: Water (H₂O), the polar nature of water allows it to effectively solvate (surround) the ions, weakening the electrostatic attraction and leading to dissolution.
In contrast, nonpolar solutes, with symmetrical electron distribution and minimal dipole moment, dissolve best in nonpolar solvents. These solvents lack significant dipole moments and rely on weaker London dispersion forces for intermolecular attractions. The similarity in intermolecular forces between solute and solvent facilitates dissolution.
Examples of Nonpolar Solutes and Solvents:
-
Solute: Oil (a mixture of hydrocarbons), characterized by nonpolar carbon-hydrogen bonds.
-
Solvent: Hexane (C₆H₁₄), a nonpolar hydrocarbon solvent. The weak London dispersion forces between oil and hexane molecules allow for mutual solubility.
-
Solute: Iodine (I₂), a nonpolar diatomic molecule.
-
Solvent: Carbon tetrachloride (CCl₄), a nonpolar solvent. Both iodine and carbon tetrachloride interact primarily through London dispersion forces.
Factors Influencing Solvent Choice Beyond "Like Dissolves Like"
While the "like dissolves like" principle serves as a useful starting point, several other factors significantly influence solvent selection:
1. Solvent Polarity: A Deeper Dive
Polarity isn't a simple on/off switch. It's a spectrum ranging from highly polar (like water) to completely nonpolar (like hexane). Many solvents fall somewhere in between, exhibiting varying degrees of polarity. The dielectric constant of a solvent provides a quantitative measure of its polarity. Higher dielectric constants indicate greater polarity. This means the solvent can effectively screen the electrostatic interactions between ions or polar molecules, facilitating dissolution.
2. Hydrogen Bonding Capacity
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that significantly affects solubility. Solvents capable of forming hydrogen bonds (like water, alcohols, and amines) readily dissolve solutes that can also participate in hydrogen bonding. This explains the high solubility of many organic molecules containing hydroxyl (-OH), amino (-NH₂), or carboxyl (-COOH) groups in polar protic solvents.
3. Solvent Viscosity
Viscosity refers to a solvent's resistance to flow. High viscosity can hinder dissolution, particularly for solids, as it makes it difficult for solvent molecules to reach and interact with the solute particles. Low-viscosity solvents generally offer faster dissolution rates.
4. Temperature
Temperature significantly impacts solubility. Increasing temperature usually enhances the solubility of solids and gases in liquids. This is because higher temperatures provide more kinetic energy, allowing solvent molecules to overcome the attractive forces holding the solute particles together.
5. Pressure
Pressure primarily affects the solubility of gases in liquids. Increasing pressure generally increases the solubility of a gas. This is explained by Henry's Law, which states that the solubility of a gas is directly proportional to the partial pressure of that gas above the liquid.
6. Solvent Toxicity and Safety
The safety and toxicity of the solvent are crucial considerations. Many common solvents are flammable, volatile, or pose health risks. Choosing a less toxic and safer solvent is essential, especially in applications involving human contact or environmental release. Proper safety precautions, including working in a well-ventilated area and using personal protective equipment (PPE), are always necessary.
Practical Guide to Solvent Selection for Different Solutes
Let's explore specific examples, illustrating the selection process for different types of solutes:
1. Ionic Compounds (e.g., NaCl, KCl):
Ionic compounds are highly polar and typically dissolve best in polar protic solvents, particularly water. Water's high dielectric constant effectively screens the electrostatic interactions between ions, facilitating their separation and dissolution. Other polar protic solvents like methanol and ethanol can also dissolve ionic compounds, though often to a lesser extent than water.
2. Polar Organic Molecules (e.g., Sugars, Alcohols):
Polar organic molecules containing hydroxyl (-OH), carbonyl (C=O), or amino (-NH₂) groups generally dissolve well in polar protic solvents. The ability to form hydrogen bonds with the solvent is a key factor. Water, methanol, ethanol, and other alcohols are excellent choices. Some polar aprotic solvents (like acetone or DMF) may also show some solubility depending on the specific structure of the polar organic molecule.
3. Nonpolar Organic Molecules (e.g., Hydrocarbons, Fats):
Nonpolar organic molecules dissolve readily in nonpolar solvents. Hydrocarbons like hexane, heptane, and cyclohexane are commonly used. Other nonpolar solvents such as toluene and benzene are also effective, although benzene is considered carcinogenic and should be handled with extreme caution. Dichloromethane is a slightly polar solvent and can sometimes dissolve some nonpolar organic molecules if stronger intermolecular forces are present.
4. Gases (e.g., CO₂, O₂):
Gas solubility depends strongly on both the nature of the gas and the solvent. Polar gases tend to dissolve better in polar solvents, while nonpolar gases tend to dissolve better in nonpolar solvents. Pressure also plays a significant role; increased pressure generally leads to increased solubility. Water is often used for dissolving polar gases, while nonpolar solvents are used for dissolving nonpolar gases.
5. Metals:
Metals generally require specific solvents that can form complexes or coordinate bonds with metal ions. These solvents often contain chelating agents or ligands that can bind to the metal atoms. Common examples include aqueous solutions containing acids or bases, or organic solvents containing coordinating ligands, such as ammonia or amines.
Conclusion: A Holistic Approach to Solvent Selection
Choosing the right solvent is a crucial step in many chemical processes. While the "like dissolves like" principle provides a basic framework, successful solvent selection requires a holistic approach. Consider the polarity, hydrogen bonding capacity, viscosity, temperature, pressure, and safety aspects of the solvent. By carefully weighing these factors, you can ensure efficient dissolution and the success of your experimental or industrial processes. Remember that careful consideration of safety and environmental impact should always be paramount when selecting a solvent. This comprehensive guide provides a solid foundation for making informed decisions in solvent selection, ensuring effective and safe outcomes in your work.
Latest Posts
Latest Posts
-
What Is 74 Degrees Celsius In Fahrenheit
May 18, 2025
-
How Many Ounces Is 156 Ml
May 18, 2025
-
90 Square Meters In Square Feet
May 18, 2025
-
How Many Ounces Is 900 Ml
May 18, 2025
-
How Many Days Is 96 Hours
May 18, 2025
Related Post
Thank you for visiting our website which covers about For Each Solute Click The Button Under The Better Solvent . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.