Fix Any Errors In These Proposed Electron Configurations
Holbox
Mar 27, 2025 · 5 min read
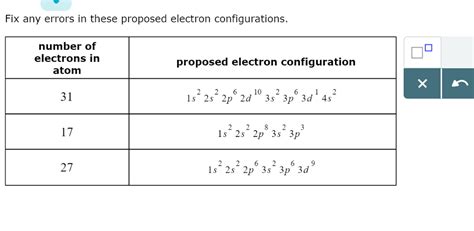
Table of Contents
- Fix Any Errors In These Proposed Electron Configurations
- Table of Contents
- Fixing Errors in Proposed Electron Configurations: A Comprehensive Guide
- Understanding the Fundamentals of Electron Configurations
- Common Errors and How to Correct Them
- 1. Incorrect Order of Orbital Filling
- 2. Violation of the Pauli Exclusion Principle
- 3. Ignoring Hund's Rule
- 4. Miscounting Electrons
- 5. Incorrect Subshell Notation
- 6. Ignoring Exceptions to the Aufbau Principle
- Advanced Techniques for Checking Electron Configurations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Fixing Errors in Proposed Electron Configurations: A Comprehensive Guide
Electron configurations describe the arrangement of electrons within an atom's orbitals. Understanding and correctly writing electron configurations is fundamental to comprehending atomic properties, chemical bonding, and reactivity. However, even experienced chemists can occasionally make mistakes. This article delves into common errors encountered when writing electron configurations and provides a systematic approach to identify and correct them. We will explore various principles governing electron configuration, examining both typical and less-common scenarios.
Understanding the Fundamentals of Electron Configurations
Before addressing common errors, let's review the basic principles:
-
Aufbau Principle: Electrons fill orbitals in order of increasing energy. This generally follows the (n+l) rule, where n is the principal quantum number and l is the azimuthal quantum number (0 for s, 1 for p, 2 for d, 3 for f). Lower (n+l) values fill first; if (n+l) values are equal, orbitals with lower n fill first.
-
Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (represented by ↑ and ↓).
-
Hund's Rule: When filling degenerate orbitals (orbitals of the same energy level, such as the three p orbitals), electrons will individually occupy each orbital before pairing up in any one orbital. Each electron in a degenerate set of orbitals will have the same spin before pairing occurs.
-
Orbital Notation: Electron configurations are often represented using orbital notation (e.g., 1s²2s²2p⁶3s¹ for Sodium). This visually depicts the filling of each subshell.
-
Electron Shell and Subshell Notation: Alternatively, a more concise notation utilizing the principal quantum number (n) and subshells (s, p, d, f) can be used (e.g., [Ne]3s¹ for Sodium, where [Ne] represents the electron configuration of Neon).
Common Errors and How to Correct Them
Now, let's dissect the frequent mistakes encountered in writing electron configurations and discuss effective strategies for correcting them.
1. Incorrect Order of Orbital Filling
A common mistake stems from incorrectly applying the Aufbau principle. Students might mistakenly fill higher energy orbitals before lower ones.
Example: An incorrect configuration for Chromium (Cr, atomic number 24) could be 1s²2s²2p⁶3s²3p⁶4s²3d⁴.
Correction: The correct configuration is 1s²2s²2p⁶3s²3p⁶4s¹3d⁵. While seemingly violating the Aufbau principle by filling 3d before 4s, this configuration is more stable due to half-filled and fully-filled subshells possessing enhanced stability. This is a crucial exception to remember. Similarly, Copper (Cu) exhibits a similar exception.
2. Violation of the Pauli Exclusion Principle
This error involves placing more than two electrons in a single orbital.
Example: An incorrect configuration for Oxygen (O, atomic number 8) might be 1s³2s²2p⁶.
Correction: The correct configuration is 1s²2s²2p⁴. Each orbital (s or p) can hold a maximum of two electrons, with opposite spins.
3. Ignoring Hund's Rule
Failing to apply Hund's rule leads to incorrect electron distribution within degenerate orbitals.
Example: An incorrect configuration for Nitrogen (N, atomic number 7) might be 1s²2s²2p² (with both electrons in the same 2p orbital).
Correction: The correct configuration is 1s²2s²2p³ (with one electron in each of the three 2p orbitals, before pairing occurs). This maximizes the total spin, resulting in a more stable configuration.
4. Miscounting Electrons
A fundamental error is simply miscounting the total number of electrons present in the configuration. This might result from overlooking electrons or adding extra electrons. Double-checking the atomic number against the total number of electrons in the configuration is crucial.
Example: A configuration for Sulfur (S, atomic number 16) might incorrectly be written with only 15 electrons.
Correction: Carefully recount the electrons in each orbital. The correct configuration for Sulfur is 1s²2s²2p⁶3s²3p⁴.
5. Incorrect Subshell Notation
Incorrectly using the subshell notations (s, p, d, f) or the principal quantum numbers (n=1, 2, 3…) can lead to errors. For example, using a 'g' subshell before filling all 'f' orbitals would be incorrect.
Example: An imaginary incorrect configuration would include a 'g' subshell before completely filling the 'f' orbitals.
Correction: Adhere to the standard order: s, p, d, f, and only introduce the next subshell after the previous one is fully occupied according to the Aufbau principle.
6. Ignoring Exceptions to the Aufbau Principle
Beyond Chromium and Copper, some other transition metals and lanthanides/actinides exhibit exceptions to the standard Aufbau filling order. These exceptions are primarily due to the relatively small energy difference between certain subshells, leading to a more stable configuration with half-filled or completely filled d or f subshells. Memorization of these exceptions is necessary for accurate configurations.
Example: Several transition metals showcase irregularities in their electron configurations, often favouring half-filled or fully-filled d orbitals.
Correction: Consult a periodic table or reliable reference for the exceptions.
Advanced Techniques for Checking Electron Configurations
Beyond the basic principles and common errors, several techniques can enhance the accuracy of your work:
-
Using Periodic Trends: Understanding periodic trends can help predict the general electron configuration of elements. For instance, elements in the same group will have similar outer electron configurations, defining their chemical behavior.
-
Utilizing Online Tools: While not replacing understanding, several online tools can check electron configurations. However, always focus on understanding the underlying principles rather than relying solely on tools.
-
Working Through Examples: Practice writing electron configurations for various elements. Start with simpler atoms and gradually progress to more complex ones. Analyze both correct and incorrect examples to sharpen your understanding.
-
Cross-checking with known properties: Once you have determined an electron configuration, consider whether the properties predicted by this configuration match the known properties of the element. For example, a configuration should appropriately predict the element's valency and its likely bonding behavior.
Conclusion
Mastering electron configurations requires a solid grasp of fundamental principles, a methodical approach, and consistent practice. By carefully reviewing the principles of the Aufbau principle, Pauli exclusion principle, and Hund's rule, and by being mindful of the common errors discussed above, one can confidently write and verify electron configurations. Remember that understanding the underlying reasons for exceptions to the rules is just as crucial as applying them. Regular practice, diligent checking, and consulting reliable resources will significantly improve accuracy and deepen your understanding of atomic structure and chemical behavior.
Latest Posts
Latest Posts
-
A Financial Advisor Schedules An Introductory Meeting
Mar 31, 2025
-
Match Each Principal Function Of Management With Its Definition
Mar 31, 2025
-
Draw The Major Organic Product Of The Following Reaction
Mar 31, 2025
-
Which Of The Following Statements About Variation Is False
Mar 31, 2025
-
The Resistance Of A Wire Depends On
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Fix Any Errors In These Proposed Electron Configurations . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
