Experiment 10 Report Sheet Vinegar Analysis
Holbox
Mar 18, 2025 · 6 min read
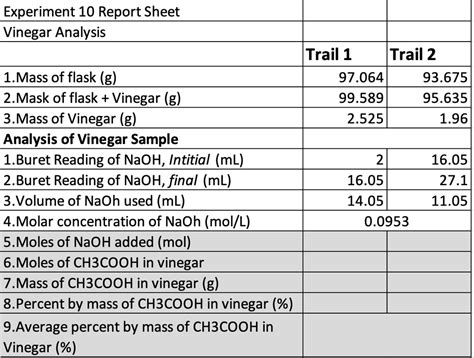
Table of Contents
Experiment 10 Report Sheet: Vinegar Analysis – A Comprehensive Guide
Vinegar, a staple in kitchens worldwide, is more than just a salad dressing ingredient. It's a dilute solution of acetic acid, a weak organic acid, in water. Understanding its composition, particularly the concentration of acetic acid, is crucial for quality control in production and for various scientific applications. This report delves into the details of a typical Experiment 10 focused on vinegar analysis, covering the procedure, results, calculations, and discussions. We'll explore the different techniques used to determine the acetic acid content, highlighting their strengths and weaknesses. This guide aims to provide a comprehensive understanding of this common experiment, equipping you with the knowledge to perform and analyze your own vinegar analysis.
Understanding the Experiment: Titration and its Importance
The most common method for determining the acetic acid concentration in vinegar is acid-base titration. This technique involves neutralizing the acetic acid in the vinegar sample with a standard solution of a strong base, usually sodium hydroxide (NaOH). By carefully measuring the volume of base required to reach the equivalence point (the point at which the acid and base have completely neutralized each other), we can calculate the concentration of acetic acid in the original vinegar sample.
The importance of this experiment extends beyond simply determining vinegar strength. It provides a hands-on learning experience in:
- Quantitative analysis: Developing skills in accurate measurements and data analysis.
- Titration techniques: Mastering the practical aspects of titration, including endpoint determination.
- Stoichiometry: Applying principles of stoichiometry to calculate the unknown concentration from the known concentration.
- Acid-base chemistry: Reinforcing understanding of acid-base reactions and equilibrium.
Materials and Methods: A Step-by-Step Guide
The materials required for this experiment are relatively simple and readily available in most chemistry labs. They include:
- Vinegar sample: A commercially available vinegar sample of unknown concentration.
- Standardized NaOH solution: A solution of sodium hydroxide with a precisely known concentration. The concentration is typically determined beforehand using a primary standard.
- Burette: A calibrated glass tube used to dispense the NaOH solution precisely.
- Erlenmeyer flask: A flask to contain the vinegar sample during the titration.
- Pipette: Used to accurately measure a specific volume of vinegar sample.
- Phenolphthalein indicator: An indicator that changes color at the endpoint of the titration.
- Wash bottle: Filled with distilled water for rinsing.
- Magnetic stirrer and stir bar: For efficient mixing during the titration (optional but recommended).
Procedure:
- Preparation: Carefully rinse the burette with the standardized NaOH solution and fill it to the zero mark. Similarly, rinse the pipette with the vinegar sample before measuring out an aliquot.
- Sample Preparation: Pipette a known volume (e.g., 25.00 mL) of the vinegar sample into the Erlenmeyer flask. Add a few drops of phenolphthalein indicator.
- Titration: Add distilled water to the flask to improve the clarity of the endpoint. Using the magnetic stirrer, slowly add the NaOH solution from the burette to the vinegar sample while constantly swirling.
- Endpoint Determination: The endpoint is reached when the solution turns a persistent faint pink color, indicating complete neutralization of the acetic acid. Record the final volume of NaOH used from the burette.
- Replicates: Repeat steps 2-4 at least three times to obtain multiple data points and improve the accuracy of the results. The closer the values are, the better the accuracy.
Calculations and Data Analysis: Unveiling the Acetic Acid Content
Once the titration is complete and the data is recorded, we can calculate the concentration of acetic acid in the vinegar sample. The following calculations are crucial:
-
Moles of NaOH used: Calculate the number of moles of NaOH used in each titration using the formula: Moles = Molarity × Volume (in Liters). Remember to convert the volume from milliliters to liters.
-
Moles of acetic acid: According to the balanced chemical equation for the neutralization reaction between acetic acid (CH₃COOH) and sodium hydroxide (NaOH):
CH₃COOH + NaOH → CH₃COONa + H₂O
The mole ratio of acetic acid to sodium hydroxide is 1:1. Therefore, the moles of acetic acid are equal to the moles of NaOH used.
-
Concentration of acetic acid: Calculate the concentration of acetic acid in the vinegar sample using the formula:
Molarity of acetic acid = Moles of acetic acid / Volume of vinegar (in Liters). Again, remember to convert the volume from milliliters to liters.
-
Percentage of acetic acid: To express the concentration as a percentage (w/v), use the following formula:
Percentage of acetic acid (w/v) = (Molarity of acetic acid × Molecular weight of acetic acid × 100) / Density of vinegar
Remember that the molecular weight of acetic acid (CH₃COOH) is 60.05 g/mol. The density of vinegar is typically close to the density of water (approximately 1 g/mL).
Results and Discussion: Interpreting the Findings
This section should present a clear summary of your results, including the volume of NaOH used in each titration, the calculated molarity of acetic acid, and the percentage of acetic acid. Include a table summarizing the data. It's crucial to compare your results to the manufacturer's stated acetic acid content on the vinegar bottle. Analyze any discrepancies and potential sources of error.
Potential Sources of Error:
- Inaccurate measurements: Errors in measuring the volume of vinegar sample or NaOH solution can significantly impact the results.
- Improper endpoint determination: The endpoint might be overshot or undershot, leading to inaccurate readings.
- Impurities in the reagents: Impurities in the vinegar sample or NaOH solution can affect the titration results.
- Calibration errors: Errors in the calibration of the burette or pipette will affect the precision of your measurements.
A thorough discussion should explore these potential sources of error and their impact on the accuracy and precision of your results. Consider what steps could be taken to minimize these errors in future experiments.
Conclusion: A Summary of Key Findings and Future Directions
The experiment successfully demonstrated the principle of acid-base titration and its application in determining the concentration of acetic acid in vinegar. The calculated percentage of acetic acid in the vinegar sample should be reported, along with a comparison to the manufacturer's claim. The discussion of potential sources of error and suggestions for improvement highlight the importance of meticulous experimental technique. Future investigations could explore different titration techniques, analyzing different types of vinegar or investigating the impact of various factors on the acetic acid concentration.
This report provides a comprehensive guide to performing and analyzing a vinegar analysis experiment. By understanding the principles involved, the procedures, calculations, and potential sources of error, you are well-equipped to conduct this experiment accurately and interpret the results effectively. Remember that meticulous attention to detail and precise measurements are essential for reliable results. The experiment serves as an excellent introduction to quantitative analysis and reinforces key concepts in acid-base chemistry and stoichiometry.
Latest Posts
Latest Posts
-
What Is The Difference Between Tough And Tuff
Mar 19, 2025
-
Locking Out Tagging Out Refers To The Practice Of
Mar 19, 2025
-
John Is Rollerblading Down A Long
Mar 19, 2025
-
Hydrolysis Of Sucrose A Disaccharide Results In
Mar 19, 2025
-
What Is The Difference Between Mutualism And Synergism
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Experiment 10 Report Sheet Vinegar Analysis . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
