Draw Two Resonance Structures Of The Cation Shown
Holbox
Mar 22, 2025 · 6 min read
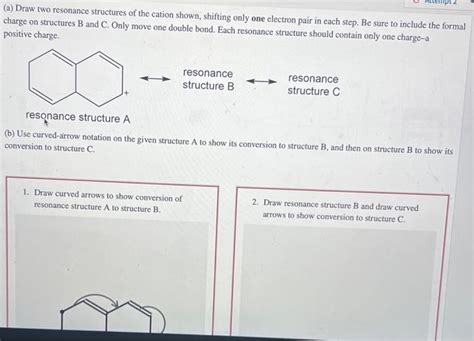
Table of Contents
- Draw Two Resonance Structures Of The Cation Shown
- Table of Contents
- Delving Deep into Resonance Structures: A Comprehensive Guide with Examples
- Understanding Resonance: The Basics
- Drawing Resonance Structures: A Step-by-Step Guide
- Example: Drawing Two Resonance Structures of a Cation
- Advanced Considerations and Complex Cases
- Limitations of Resonance Theory
- Applications of Resonance in Chemistry
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Delving Deep into Resonance Structures: A Comprehensive Guide with Examples
Resonance structures are a crucial concept in organic chemistry, representing the delocalization of electrons within a molecule or ion. Understanding resonance is essential for predicting molecular properties like stability, reactivity, and bond lengths. This article will thoroughly explore resonance structures, focusing specifically on drawing two resonance structures for a given cation, providing a detailed explanation of the process and underlying principles. We'll also touch upon the limitations of resonance theory and its practical applications.
Understanding Resonance: The Basics
Before diving into drawing resonance structures, let's establish a firm grasp of the fundamental principles. Resonance describes the phenomenon where a single Lewis structure is insufficient to accurately represent the actual distribution of electrons within a molecule or ion. Instead, a molecule or ion exists as a hybrid of multiple contributing structures, called resonance structures or canonical forms. These structures differ only in the placement of electrons (specifically pi electrons and lone pairs), not in the arrangement of atoms.
Key Characteristics of Resonance Structures:
- Same Atom Connectivity: All resonance structures must have the same arrangement of atoms. Only the electron distribution changes.
- Equivalent Energy (Ideally): While resonance structures contribute differently to the resonance hybrid, ideally, they should be of similar energy. Structures with significant differences in energy contribute less significantly to the hybrid.
- Formal Charges: Resonance structures may have different formal charges on atoms. The sum of formal charges across all atoms remains constant across all resonance structures.
- Octet Rule (Mostly): While aiming for octets on atoms (especially for second-row elements like carbon, nitrogen, and oxygen), it’s crucial to remember that resonance structures may show atoms with fewer than eight electrons (especially in carbocations).
Drawing Resonance Structures: A Step-by-Step Guide
Drawing accurate resonance structures is a skill honed through practice. Here’s a systematic approach:
-
Draw the Lewis Structure: Begin by drawing the Lewis structure of the molecule or ion. This involves identifying the central atom, connecting atoms with single bonds, and distributing valence electrons to satisfy the octet rule (where possible).
-
Identify Pi Electrons and Lone Pairs: Locate pi electrons (those involved in double or triple bonds) and lone pairs of electrons. These are the electrons that can be delocalized through resonance.
-
Move Electrons: Systematically move pi electrons and lone pairs to create new valid Lewis structures. Remember, only electrons move; atoms remain in the same positions. Arrows are used to show the movement of electron pairs. A curved arrow starts from the electron pair's original location and points to its new location.
-
Check Formal Charges: After each electron movement, calculate the formal charge on each atom in the new structure. The sum of formal charges should be consistent across all resonance structures.
-
Evaluate the Resonance Structures: Determine the relative contributions of each resonance structure to the resonance hybrid. Structures with fewer formal charges, those with negative charges on more electronegative atoms, and those with complete octets (where possible) typically contribute more significantly.
Example: Drawing Two Resonance Structures of a Cation
Let’s illustrate this process with an example. Consider the allyl cation (CH₂=CH-CH₂⁺).
Step 1: Lewis Structure: The initial Lewis structure shows a double bond between the first two carbons and a positive charge on the terminal carbon.
H₂C=CH-CH₂⁺
Step 2: Identify Movable Electrons: The pi electrons in the double bond are the electrons that can be delocalized.
Step 3: Move Electrons: We can move the pi electrons from the double bond to form a new double bond between the second and third carbon, resulting in a positive charge on the first carbon.
Resonance Structure 1:
H₂C=CH-CH₂⁺
Resonance Structure 2:
H₂C⁺-CH=CH₂
The curved arrow shows the movement of the pi electron pair. Notice that in both structures, the atom connectivity remains the same; only the position of the double bond and the positive charge changes.
Step 4: Check Formal Charges: Both structures show one carbon with a +1 formal charge, consistent with the overall cationic charge.
Step 5: Evaluate Structures: Both resonance structures contribute equally to the resonance hybrid as they are energetically equivalent. The actual structure of the allyl cation is a hybrid of these two structures, with the positive charge delocalized across both terminal carbons. The carbon-carbon bond lengths are somewhere between a single and a double bond, reflecting the delocalized nature of the pi electrons.
Advanced Considerations and Complex Cases
While the allyl cation is a relatively simple example, resonance can involve more complex scenarios. Here are a few considerations:
- Aromatic Systems: Aromatic compounds, like benzene, exhibit extensive resonance delocalization. Benzene has multiple resonance structures, all contributing equally to its exceptional stability.
- Multiple Resonance Structures: Some molecules exhibit many resonance structures, and assessing their relative contributions can be complex.
- Steric Effects: In some cases, steric hindrance might influence the relative contribution of resonance structures.
- Electron-Donating and Withdrawing Groups: The presence of electron-donating or electron-withdrawing groups can significantly alter the stability and relative contributions of resonance structures.
Limitations of Resonance Theory
It is crucial to understand that resonance structures are merely representations; they don't represent distinct, separate molecules. The actual molecule exists as a resonance hybrid, which is a weighted average of all contributing structures. The hybrid is more stable than any individual resonance structure. It is also important to remember that resonance structures are not transition states in a reaction mechanism, nor are they intermediate structures.
Applications of Resonance in Chemistry
Understanding resonance has broad applications in chemistry:
- Predicting Reactivity: Resonance stabilization can significantly affect a molecule's reactivity. Highly stabilized molecules are less reactive than those with less resonance stabilization.
- Explaining Bond Lengths and Bond Orders: Resonance leads to bond lengths and bond orders that are intermediate between typical single and double bonds.
- Spectroscopy: Resonance structures influence a molecule's spectroscopic properties, including NMR and UV-Vis spectroscopy.
- Drug Design: Understanding resonance is essential in designing drugs. Resonance can significantly influence the binding affinity and activity of drug molecules.
Conclusion
Resonance is a fundamental concept that significantly impacts our understanding of molecular structure and reactivity. Mastering the skill of drawing and analyzing resonance structures is critical for organic chemists. This article provided a detailed guide to understanding and applying resonance theory, illustrated with a clear example and highlighted some advanced considerations and limitations. By consistently practicing and applying the principles discussed, one can develop the expertise necessary to predict and interpret the behavior of molecules in various chemical contexts. Remember, the more you practice drawing and analyzing resonance structures, the more intuitive this important concept will become.
Latest Posts
Latest Posts
-
Which Of The Following Is A Mission Area
Mar 24, 2025
-
Rn Targeted Medical Surgical Respiratory Online Practice 2023
Mar 24, 2025
-
The Division Of The Cytoplasm Is Called
Mar 24, 2025
-
Select Characteristics Exhibited By All Bacteria
Mar 24, 2025
-
What Is The Difference Between Microeconomics And Macroeconomics
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Draw Two Resonance Structures Of The Cation Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
