Draw The Product Of The Reaction Shown Between Propanoyl Chloride
Holbox
Mar 23, 2025 · 6 min read
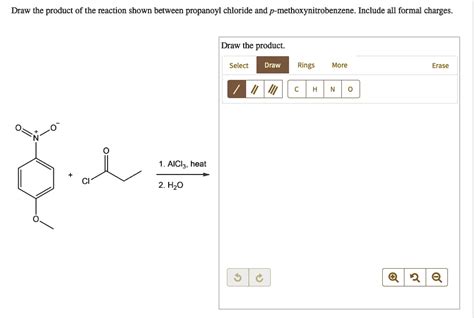
Table of Contents
- Draw The Product Of The Reaction Shown Between Propanoyl Chloride
- Table of Contents
- Drawing the Product of the Reaction Between Propanoyl Chloride and Various Reagents: A Comprehensive Guide
- Understanding Nucleophilic Acyl Substitution
- Reactions of Propanoyl Chloride with Various Nucleophiles
- 1. Reaction with Water (Hydrolysis): Formation of Propionic Acid
- 2. Reaction with Alcohols: Formation of Esters
- 3. Reaction with Ammonia (NH3) and Amines (RNH2): Formation of Amides
- 4. Reaction with Grignard Reagents (RMgX): Formation of Ketones
- 5. Reaction with Lithium Aluminum Hydride (LiAlH4): Formation of Primary Alcohol
- 6. Reaction with Carboxylic Acids: Formation of Acid Anhydrides
- Factors Affecting Reaction Outcomes
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Product of the Reaction Between Propanoyl Chloride and Various Reagents: A Comprehensive Guide
Propanoyl chloride, also known as propionyl chloride, is a reactive acyl halide that readily undergoes nucleophilic acyl substitution reactions. Understanding the products of these reactions is crucial for organic chemistry students and professionals alike. This comprehensive guide will explore the products formed when propanoyl chloride reacts with a variety of nucleophiles, detailing the mechanisms and highlighting important considerations. We'll delve into the reactions' specifics, emphasizing the key structural changes and the resulting compounds.
Understanding Nucleophilic Acyl Substitution
Before we delve into specific reactions, let's review the general mechanism of nucleophilic acyl substitution. This reaction proceeds through a tetrahedral intermediate, a key step that dictates the final product. The mechanism typically involves these steps:
-
Nucleophilic attack: The nucleophile (Nu⁻) attacks the electrophilic carbonyl carbon of the propanoyl chloride. This creates a negatively charged tetrahedral intermediate.
-
Leaving group departure: The chloride ion (Cl⁻), a good leaving group, departs, regenerating the carbonyl group. This step often involves the concomitant protonation of the oxygen atom.
-
Product formation: The resulting product is an ester, amide, carboxylic acid, or another derivative, depending on the nature of the nucleophile.
Reactions of Propanoyl Chloride with Various Nucleophiles
Let's examine the products formed when propanoyl chloride reacts with a range of nucleophiles. We will consider the structure of the resulting products and the reaction conditions which might influence the yield and selectivity.
1. Reaction with Water (Hydrolysis): Formation of Propionic Acid
When propanoyl chloride reacts with water, it undergoes hydrolysis to form propionic acid and hydrochloric acid. Water acts as the nucleophile, attacking the carbonyl carbon. The chloride ion leaves, and subsequent proton transfer leads to the formation of propionic acid.
CH3CH2COCl + H2O → CH3CH2COOH + HCl
Mechanism: The water molecule attacks the carbonyl carbon, forming a tetrahedral intermediate. The chloride ion leaves, and a proton is transferred from the positively charged oxygen to a water molecule, resulting in propionic acid and HCl.
2. Reaction with Alcohols: Formation of Esters
Reaction with alcohols (ROH) results in the formation of esters. This reaction is commonly known as esterification. The alcohol acts as the nucleophile, attacking the carbonyl carbon. The chloride ion leaves, yielding an ester and HCl.
CH3CH2COCl + ROH → CH3CH2COOR + HCl
Mechanism: Similar to hydrolysis, the alcohol attacks the carbonyl carbon, creating a tetrahedral intermediate. The chloride leaves, followed by proton transfer, resulting in the formation of an ester and HCl. The specific ester formed depends entirely on the alcohol used (e.g., methanol yields methyl propionate, ethanol yields ethyl propionate, etc.).
Example: The reaction of propanoyl chloride with methanol (CH3OH) produces methyl propionate (CH3CH2COOCH3) and HCl.
3. Reaction with Ammonia (NH3) and Amines (RNH2): Formation of Amides
Reaction with ammonia or primary amines produces amides. The nitrogen atom in ammonia or the amine acts as the nucleophile, attacking the carbonyl carbon. The chloride ion leaves, and subsequent proton transfer yields an amide and HCl.
CH3CH2COCl + NH3 → CH3CH2CONH2 + HCl (with ammonia, producing propanamide)
CH3CH2COCl + RNH2 → CH3CH2CONHR + HCl (with a primary amine, producing a substituted amide)
Mechanism: The nitrogen atom attacks the carbonyl carbon, forming a tetrahedral intermediate. The chloride ion leaves, and proton transfer results in the amide and HCl. The R group on the amine determines the specific amide structure.
Example: The reaction of propanoyl chloride with methylamine (CH3NH2) produces N-methylpropanamide (CH3CH2CONHCH3) and HCl.
4. Reaction with Grignard Reagents (RMgX): Formation of Ketones
Grignard reagents (RMgX), where R is an alkyl or aryl group and X is a halide, are powerful nucleophiles. Their reaction with propanoyl chloride yields a ketone after an acidic workup. This reaction involves two steps: the initial nucleophilic attack by the Grignard reagent followed by hydrolysis.
CH3CH2COCl + RMgX → CH3CH2COR + MgXCl (followed by hydrolysis to remove MgXCl)
Mechanism: The Grignard reagent acts as a nucleophile attacking the carbonyl carbon, forming a tetrahedral intermediate. The chloride ion leaves. The resulting organomagnesium compound then undergoes hydrolysis with dilute acid to produce the ketone. The R group from the Grignard reagent determines the structure of the resulting ketone.
Example: The reaction of propanoyl chloride with methylmagnesium bromide (CH3MgBr) would initially yield a magnesium alkoxide intermediate, which upon acidic workup, would produce 3-pentanone (CH3CH2COCH3).
5. Reaction with Lithium Aluminum Hydride (LiAlH4): Formation of Primary Alcohol
Lithium aluminum hydride (LiAlH4) is a strong reducing agent. It reduces propanoyl chloride to a primary alcohol, specifically propan-1-ol. This is a significantly different reaction than the previous nucleophilic acyl substitutions, as LiAlH4 is not strictly a nucleophile in this context; instead, it acts as a hydride donor.
CH3CH2COCl + LiAlH4 → CH3CH2CH2OH + (byproducts)
Mechanism: This reaction involves multiple steps and the detailed mechanism is complex, involving hydride transfer from LiAlH4 to the carbonyl group. The reduction proceeds via an alkoxide intermediate which is then hydrolyzed to give the alcohol.
6. Reaction with Carboxylic Acids: Formation of Acid Anhydrides
Reaction with carboxylic acids leads to the formation of acid anhydrides. This requires a strong dehydrating agent such as pyridine to drive the equilibrium towards anhydride formation.
CH3CH2COCl + RCOOH → CH3CH2CO-O-COR + HCl
Mechanism: The carboxylate ion (RCOO⁻) acts as the nucleophile, attacking the carbonyl carbon of propanoyl chloride. Chloride ion leaves, followed by proton transfer, leading to the acid anhydride.
Example: The reaction of propanoyl chloride with acetic acid (CH3COOH) yields propionic acetic anhydride.
Factors Affecting Reaction Outcomes
Several factors can influence the success and outcome of these reactions:
-
Solvent: The choice of solvent plays a crucial role in solubility and reaction rate. Polar aprotic solvents are often preferred for nucleophilic acyl substitution reactions.
-
Temperature: Generally, higher temperatures accelerate the reaction rate. However, extremely high temperatures might lead to side reactions or decomposition.
-
Steric hindrance: Bulky groups around the carbonyl carbon or the nucleophile can hinder the reaction, slowing it down or even preventing it entirely.
-
Reaction stoichiometry: The ratio of reactants influences the product yield. For example, using an excess of nucleophile ensures that all the propanoyl chloride reacts.
Conclusion
Propanoyl chloride is a versatile reagent that participates in a variety of nucleophilic acyl substitution reactions. Understanding the mechanism of these reactions and the factors that influence them allows for the prediction and synthesis of a wide range of organic compounds, including esters, amides, carboxylic acids, ketones, and acid anhydrides. The reaction with lithium aluminum hydride provides a route to reduction, highlighting the diverse reactivity of this acyl halide. This guide provides a comprehensive overview, highlighting the importance of careful consideration of reaction conditions and nucleophile selection for successful synthesis. Remember always to prioritize safety in the lab when working with reactive chemicals.
Latest Posts
Latest Posts
-
A Sales Rep Is Displaying His Companys Newest Smartwatches
Mar 26, 2025
-
Consider The Following Scenarios Which Behaviors Must Be Reported
Mar 26, 2025
-
Which Of The Following Is Correct
Mar 26, 2025
-
Dna Replication Is Called Semiconservative Because
Mar 26, 2025
-
Rank The Following Quantities In Order Of Decreasing Distance
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Draw The Product Of The Reaction Shown Between Propanoyl Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
