Draw The Missing Organic Compounds. Do Not Draw Inorganic By-products.
Holbox
Mar 29, 2025 · 5 min read
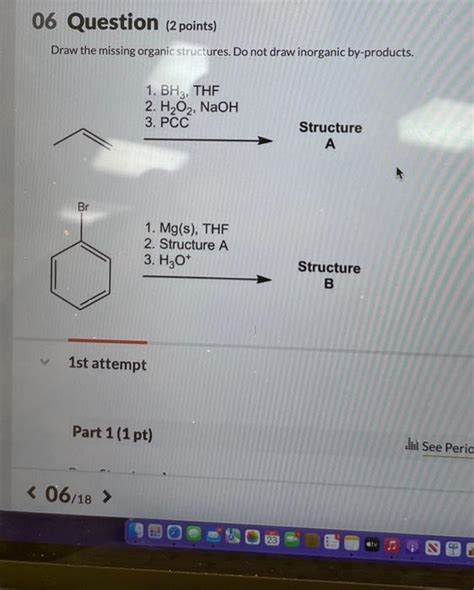
Table of Contents
- Draw The Missing Organic Compounds. Do Not Draw Inorganic By-products.
- Table of Contents
- Draw the Missing Organic Compounds: A Comprehensive Guide
- Understanding Reaction Mechanisms: The Key to Success
- Common Reaction Types and Their Mechanisms
- Predicting Products: A Step-by-Step Approach
- Example 1: Electrophilic Addition to Alkenes
- Example 2: Nucleophilic Substitution (SN2 Reaction)
- Example 3: Dehydration of Alcohols
- Example 4: Oxidation of Alcohols
- Example 5: Esterification
- Example 6: Aldol Condensation
- Advanced Scenarios and Considerations
- Practicing and Improving Your Skills
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Draw the Missing Organic Compounds: A Comprehensive Guide
Drawing organic compounds can be a daunting task, especially when dealing with incomplete reaction schemes or synthesis pathways. This comprehensive guide will walk you through the process of identifying and drawing the missing organic compounds in various reactions, focusing solely on the organic products and omitting inorganic by-products. We'll cover several reaction types, providing examples and explanations to enhance your understanding. Remember, accurately predicting reaction products requires a solid understanding of organic chemistry principles, including reaction mechanisms and functional group transformations.
Understanding Reaction Mechanisms: The Key to Success
Before tackling specific examples, let's establish the fundamental importance of understanding reaction mechanisms. A reaction mechanism is a step-by-step description of how a reaction proceeds, detailing the movement of electrons and the formation and breaking of bonds. This understanding is crucial because it allows you to predict the structure of the products formed. Without grasping the mechanism, you're essentially guessing, leading to potential inaccuracies.
Common Reaction Types and Their Mechanisms
Several fundamental reaction types are frequently encountered in organic chemistry. Mastering these is essential for successfully drawing missing organic compounds. These include:
-
Addition Reactions: These involve the addition of one molecule to another, often resulting in the formation of a larger molecule. Common examples include electrophilic addition to alkenes and nucleophilic addition to carbonyl compounds.
-
Elimination Reactions: These reactions involve the removal of atoms or groups from a molecule, often resulting in the formation of a double or triple bond. Dehydration of alcohols and dehydrohalogenation of alkyl halides are common examples.
-
Substitution Reactions: These involve the replacement of one atom or group with another. Common types include SN1, SN2, electrophilic aromatic substitution, and nucleophilic acyl substitution.
-
Rearrangement Reactions: These involve the reorganization of atoms within a molecule, leading to a structural isomer of the starting material. Common rearrangements include Claisen rearrangement and the Wagner-Meerwein rearrangement.
Predicting Products: A Step-by-Step Approach
Let's illustrate the process of predicting the missing organic compounds with several examples. Remember, always consider the reaction mechanism to make accurate predictions.
Example 1: Electrophilic Addition to Alkenes
Consider the reaction of ethene with bromine (Br₂). This is a classic example of electrophilic addition.
Reactants: Ethene (CH₂=CH₂) + Bromine (Br₂)
Mechanism: The bromine molecule acts as an electrophile, attacking the electron-rich double bond of ethene. This forms a cyclic bromonium ion intermediate. A bromide ion then attacks the bromonium ion, leading to the formation of the final product.
Missing Organic Compound: 1,2-dibromoethane (BrCH₂CH₂Br)
Example 2: Nucleophilic Substitution (SN2 Reaction)
Consider the reaction of chloromethane (CH₃Cl) with sodium hydroxide (NaOH). This is a classic SN2 reaction.
Reactants: Chloromethane (CH₃Cl) + Sodium Hydroxide (NaOH)
Mechanism: The hydroxide ion (OH⁻) acts as a nucleophile, attacking the carbon atom bonded to the chlorine atom. This leads to a backside attack, inverting the stereochemistry at the carbon center (if chiral). The chlorine atom leaves as a chloride ion.
Missing Organic Compound: Methanol (CH₃OH)
Example 3: Dehydration of Alcohols
Consider the dehydration of ethanol (CH₃CH₂OH) using a strong acid catalyst like sulfuric acid (H₂SO₄).
Reactants: Ethanol (CH₃CH₂OH)
Mechanism: The acid protonates the hydroxyl group, making it a better leaving group. Water is then eliminated, forming a carbocation intermediate. A proton is then removed from a neighboring carbon, forming a double bond.
Missing Organic Compound: Ethene (CH₂=CH₂)
Example 4: Oxidation of Alcohols
Consider the oxidation of a secondary alcohol, like propan-2-ol (CH₃CH(OH)CH₃), using an oxidizing agent like chromic acid (H₂CrO₄).
Reactants: Propan-2-ol (CH₃CH(OH)CH₃)
Mechanism: The chromic acid oxidizes the secondary alcohol, removing two hydrogen atoms from the carbon atom bearing the hydroxyl group.
Missing Organic Compound: Propanone (CH₃COCH₃)
Example 5: Esterification
Consider the reaction between ethanoic acid (CH₃COOH) and ethanol (CH₃CH₂OH) to form an ester.
Reactants: Ethanoic acid (CH₃COOH) + Ethanol (CH₃CH₂OH)
Mechanism: This is an acid-catalyzed reaction where the carboxylic acid group reacts with the alcohol group. Water is eliminated.
Missing Organic Compound: Ethyl ethanoate (CH₃COOCH₂CH₃)
Example 6: Aldol Condensation
Consider the base-catalyzed aldol condensation of two molecules of ethanal (CH₃CHO).
Reactants: Ethanal (CH₃CHO)
Mechanism: A base deprotonates one molecule of ethanal, forming a nucleophilic enolate. This enolate attacks another molecule of ethanal, forming an aldol intermediate. Dehydration then occurs, forming an α,β-unsaturated aldehyde.
Missing Organic Compound: But-2-enal (CH₃CH=CHCHO)
Advanced Scenarios and Considerations
As you progress in organic chemistry, you'll encounter more complex reactions and scenarios. Here are some points to consider:
-
Stereochemistry: Pay close attention to stereochemistry. Reactions like SN2 inverts stereochemistry, while SN1 can lead to racemization.
-
Regioselectivity: In reactions with multiple possible sites of reaction, predict which site is favored. Markovnikov's rule is a useful guideline for electrophilic addition to alkenes.
-
Multiple Products: Some reactions can yield multiple products. Predict the major and minor products based on the reaction mechanism and relative stability of the products.
-
Reaction Conditions: The reaction conditions (temperature, solvent, catalyst) can significantly influence the outcome of a reaction. Be sure to consider these factors.
Practicing and Improving Your Skills
Consistent practice is key to mastering the art of drawing missing organic compounds. Work through numerous examples, focusing on understanding the reaction mechanisms. Use online resources, textbooks, and practice problems to hone your skills.
Conclusion
Drawing the missing organic compounds in reactions requires a thorough understanding of organic chemistry principles and reaction mechanisms. By carefully analyzing the reactants, understanding the reaction mechanism, and applying relevant principles, you can accurately predict and draw the missing organic compounds. Remember to always consider stereochemistry, regioselectivity, and the possibility of multiple products. Consistent practice and a dedication to understanding reaction mechanisms are the keys to success.
Latest Posts
Latest Posts
-
Las Nuevas Normas Astm Para El Calzado Especifican Que Debe
Apr 02, 2025
-
Correctly Label The Pectoral And Brachial Muscles
Apr 02, 2025
-
Drag Each Label To The Type Of Gland It Describes
Apr 02, 2025
-
Name The Membranous Encasement Surrounding The Brain
Apr 02, 2025
-
Select The Statements About The K T Boundary That Are True
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Draw The Missing Organic Compounds. Do Not Draw Inorganic By-products. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
