Draw The Major Product Of This Reaction. Ignore Inorganic Byproducts.
Holbox
Mar 13, 2025 · 6 min read
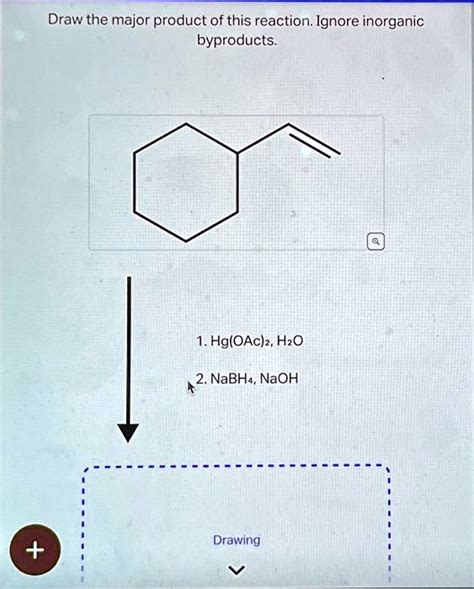
Table of Contents
- Draw The Major Product Of This Reaction. Ignore Inorganic Byproducts.
- Table of Contents
- Draw the Major Product of This Reaction: A Comprehensive Guide to Organic Chemistry Reaction Prediction
- Understanding Reaction Mechanisms: The Key to Prediction
- 1. SN1 and SN2 Reactions: Nucleophilic Substitution
- 2. E1 and E2 Reactions: Elimination Reactions
- 3. Addition Reactions: Alkenes and Alkynes
- 4. Electrophilic Aromatic Substitution
- Factors Influencing Product Formation
- Advanced Considerations: Equilibrium and Kinetic Control
- Improving Your Predictive Skills: A Practical Approach
- Conclusion
- Latest Posts
- Related Post
Draw the Major Product of This Reaction: A Comprehensive Guide to Organic Chemistry Reaction Prediction
Predicting the major product of an organic chemistry reaction is a fundamental skill for any student or professional in the field. This seemingly simple task requires a deep understanding of reaction mechanisms, functional group reactivity, and the principles of thermodynamics and kinetics. This article will delve into the process of predicting the major product, focusing on various reaction types and the factors influencing product formation. We'll explore common pitfalls and offer strategies for improving your predictive abilities. Remember, we will ignore inorganic byproducts throughout this discussion.
Understanding Reaction Mechanisms: The Key to Prediction
The foundation of accurately predicting reaction products lies in a thorough grasp of the reaction mechanism. A mechanism outlines the step-by-step process of bond breaking and bond formation, detailing the movement of electrons and the formation of intermediate species. Different mechanisms lead to different products. Let's look at some common reaction types and their associated mechanisms:
1. SN1 and SN2 Reactions: Nucleophilic Substitution
Nucleophilic substitution reactions involve the replacement of a leaving group by a nucleophile. Two major mechanisms govern these reactions:
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism proceeds via a carbocation intermediate. The rate-determining step is the unimolecular dissociation of the leaving group, making the reaction rate dependent only on the concentration of the substrate. SN1 reactions favor tertiary substrates due to the greater stability of the resulting tertiary carbocation. Rearrangements are common in SN1 reactions.
-
SN2 (Substitution Nucleophilic Bimolecular): This mechanism involves a concerted reaction where the nucleophile attacks the substrate simultaneously as the leaving group departs. The rate depends on the concentration of both the substrate and the nucleophile. SN2 reactions favor primary substrates due to steric hindrance effects. Inversion of configuration is observed in SN2 reactions.
Example: Consider the reaction between 2-bromobutane and sodium methoxide (NaOCH3). Knowing that methoxide is a strong nucleophile and 2-bromobutane is a secondary substrate, we anticipate competition between SN1 and SN2 pathways. However, since the reaction likely occurs under relatively non-polar conditions (methoxide in a protic solvent might favor SN2 more), an SN2 reaction would be favored, leading to the major product: 2-methoxybutane with inversion of stereochemistry at the carbon center.
2. E1 and E2 Reactions: Elimination Reactions
Elimination reactions involve the removal of a leaving group and a proton from adjacent carbon atoms, resulting in the formation of a double bond (alkene). Like nucleophilic substitutions, two main mechanisms exist:
-
E1 (Elimination Unimolecular): This mechanism also proceeds through a carbocation intermediate. The rate-determining step is the unimolecular formation of the carbocation. E1 reactions are favored by tertiary substrates and are often competitive with SN1 reactions. Zaitsev's rule often dictates which alkene is the major product (more substituted alkene).
-
E2 (Elimination Bimolecular): This is a concerted mechanism where the base abstracts a proton and the leaving group departs simultaneously. The reaction rate depends on both the substrate and the base concentration. E2 reactions often follow Zaitsev's rule, favoring the formation of the more substituted alkene, although steric factors can sometimes override this preference. Anti-periplanar geometry is preferred for E2 elimination.
Example: The reaction of 2-bromo-2-methylbutane with potassium tert-butoxide (t-BuOK) under basic conditions. The tert-butoxide is a bulky strong base, favoring an E2 mechanism. The major product will be the more substituted alkene, 2-methyl-2-butene, according to Zaitsev’s rule.
3. Addition Reactions: Alkenes and Alkynes
Addition reactions involve the addition of atoms or groups to a multiple bond (alkene or alkyne). The regioselectivity and stereoselectivity of these reactions are crucial in predicting the major product. Markovnikov's rule often guides the prediction of regioselectivity for electrophilic addition to alkenes.
Example: The addition of HBr to propene. Markovnikov's rule predicts that the proton will add to the less substituted carbon, resulting in the formation of 2-bromopropane as the major product. This is because the more stable secondary carbocation is formed as an intermediate.
4. Electrophilic Aromatic Substitution
Electrophilic aromatic substitution reactions involve the replacement of a hydrogen atom on an aromatic ring with an electrophile. The directing effects of substituents on the aromatic ring are crucial for predicting the major product. Activating groups (e.g., -OH, -NH2) are ortho/para directing, while deactivating groups (e.g., -NO2, -COOH) are meta directing.
Example: Nitration of toluene. The methyl group is an activating, ortho/para directing group. Therefore, the major products will be ortho-nitrotoluene and para-nitrotoluene, with para-nitrotoluene usually being slightly more favored due to steric hindrance in the ortho position.
Factors Influencing Product Formation
Several factors can influence the outcome of a reaction and determine the major product:
-
Substrate Structure: The structure of the starting material significantly impacts reactivity and selectivity. Steric hindrance, stability of intermediates (e.g., carbocations), and the presence of functional groups all play a role.
-
Reagent Strength and Type: The nature of the reagents (nucleophile, electrophile, base) significantly affects the reaction pathway and the preference for SN1/SN2, E1/E2, etc. Strong bases favor elimination, while weaker bases may favor substitution. The strength and size of a nucleophile can influence the product formation as well.
-
Solvent Effects: The solvent can significantly influence reaction rates and selectivity. Polar protic solvents favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 reactions.
-
Temperature: Temperature can affect the relative rates of competing reactions. Higher temperatures often favor elimination reactions over substitution reactions.
-
Stereochemistry: The stereochemistry of the starting material affects the stereochemistry of the products. SN2 reactions lead to inversion of configuration, while SN1 reactions often lead to racemization.
Advanced Considerations: Equilibrium and Kinetic Control
In some reactions, multiple products are possible, and the major product is determined by whether the reaction is under kinetic or thermodynamic control.
-
Kinetic Control: The major product is the one formed faster. This is often the case in reactions at lower temperatures where the activation energy barrier is more significant.
-
Thermodynamic Control: The major product is the most stable product. This is often observed at higher temperatures where sufficient energy is available to overcome the activation energy barriers and allow the reaction to reach equilibrium.
Improving Your Predictive Skills: A Practical Approach
Predicting the major product of a reaction requires practice and a systematic approach. Here’s a helpful strategy:
-
Identify the Functional Groups: Determine the key functional groups present in the starting material and reagents.
-
Determine the Reaction Type: Based on the functional groups and reagents, identify the likely reaction type (SN1, SN2, E1, E2, addition, etc.).
-
Consider Reaction Mechanisms: Draw out the mechanism step-by-step, identifying intermediates and transition states.
-
Analyze the Factors Influencing Product Formation: Consider the factors discussed earlier (substrate structure, reagent strength, solvent, temperature) to determine which factors might favor specific products.
-
Apply Rules and Principles: Use rules such as Markovnikov's rule, Zaitsev's rule, and the directing effects of substituents to predict regioselectivity and stereoselectivity.
-
Evaluate Stability of Products: Consider the stability of the potential products (e.g., more substituted alkenes are generally more stable).
Conclusion
Predicting the major product of an organic chemistry reaction is a challenging but rewarding skill. By understanding reaction mechanisms, applying relevant principles, and considering the various influencing factors, you can significantly improve your ability to accurately predict reaction outcomes. Remember to practice consistently and learn from your mistakes. This comprehensive guide provides a strong foundation for mastering this essential aspect of organic chemistry. Continuous learning and exploration of diverse reaction scenarios will further hone your predictive capabilities. The more problems you work through, the more intuitive this process will become.
Latest Posts
Related Post
Thank you for visiting our website which covers about Draw The Major Product Of This Reaction. Ignore Inorganic Byproducts. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.