Draw The Major Product Of The Substitution Reaction Shown Below
Holbox
Mar 24, 2025 · 6 min read
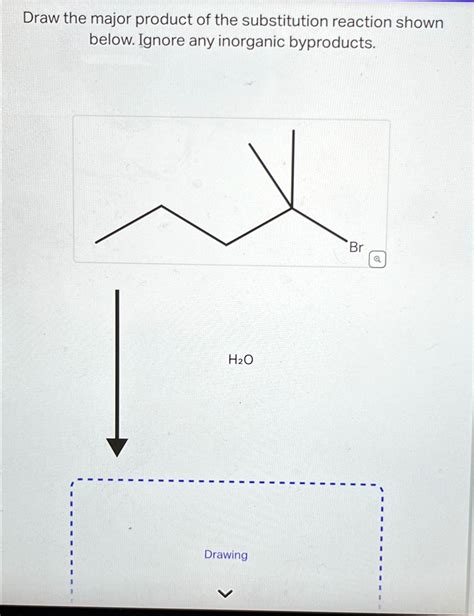
Table of Contents
- Draw The Major Product Of The Substitution Reaction Shown Below
- Table of Contents
- Drawing the Major Product of Substitution Reactions: A Comprehensive Guide
- Understanding Substitution Reactions
- SN1 Reactions: A Unimolecular Pathway
- Step 1: Formation of a Carbocation
- Step 2: Nucleophilic Attack
- Factors Favoring SN1 Reactions
- Predicting the Major Product in SN1 Reactions
- SN2 Reactions: A Bimolecular Pathway
- The Concerted Mechanism
- Factors Favoring SN2 Reactions
- Predicting the Major Product in SN2 Reactions
- Comparing SN1 and SN2 Reactions: A Summary Table
- Illustrative Examples
- Advanced Considerations: Steric Hindrance and Leaving Group Ability
- Conclusion: Mastering Substitution Reactions
- Latest Posts
- Latest Posts
- Related Post
Drawing the Major Product of Substitution Reactions: A Comprehensive Guide
Substitution reactions are fundamental in organic chemistry, forming the backbone of countless synthetic pathways. Understanding how to predict the major product of a substitution reaction is crucial for success in this field. This comprehensive guide will delve into the intricacies of substitution reactions, focusing on predicting the major product, considering various factors that influence the outcome. We'll cover both SN1 and SN2 mechanisms, exploring the nuances of each and providing clear examples to solidify your understanding.
Understanding Substitution Reactions
Substitution reactions, in their simplest form, involve the replacement of one atom or group (a leaving group) in a molecule with another atom or group (a nucleophile). This process fundamentally alters the structure and properties of the original molecule. There are two primary mechanisms governing these reactions: SN1 (substitution nucleophilic unimolecular) and SN2 (substitution nucleophilic bimolecular). The mechanism followed dictates the stereochemistry and the regiochemistry of the product.
SN1 Reactions: A Unimolecular Pathway
SN1 reactions proceed through a two-step mechanism.
Step 1: Formation of a Carbocation
The first step involves the departure of the leaving group, creating a carbocation intermediate. This step is the rate-determining step, hence the "unimolecular" designation (rate depends only on the concentration of the substrate). The stability of this carbocation intermediate is paramount in determining the outcome of the reaction. More stable carbocations (tertiary > secondary > primary > methyl) are formed more readily.
Step 2: Nucleophilic Attack
In the second step, the nucleophile attacks the carbocation, forming a new bond. This step is generally fast. Because the carbocation is planar, the nucleophile can attack from either side, leading to a racemic mixture of products if the starting material was chiral.
Factors Favoring SN1 Reactions
- Weak Nucleophiles: SN1 reactions are favored by weak nucleophiles, as strong nucleophiles would prefer the faster SN2 pathway.
- Tertiary, Secondary, or Benzylic/Allylic Substrates: These substrates readily form relatively stable carbocations. Primary substrates rarely undergo SN1 reactions.
- Protic Solvents: Protic solvents (like water or alcohols) help stabilize the carbocation intermediate and the leaving group.
Predicting the Major Product in SN1 Reactions
In SN1 reactions, the major product is determined primarily by the stability of the carbocation intermediate. If multiple carbocations can form (e.g., through rearrangement), the most stable carbocation will be the predominant intermediate, leading to the major product. For example, in a tertiary substrate, the reaction will favor the formation of the tertiary substituted product. However, if rearrangements are possible (hydride or alkyl shifts), these rearrangements will occur to produce an even more stable carbocation.
SN2 Reactions: A Bimolecular Pathway
SN2 reactions proceed through a concerted, one-step mechanism.
The Concerted Mechanism
The nucleophile attacks the substrate from the backside of the leaving group, simultaneously displacing the leaving group. This backside attack leads to inversion of configuration at the stereocenter (Walden inversion).
Factors Favoring SN2 Reactions
- Strong Nucleophiles: SN2 reactions require strong nucleophiles to facilitate the backside attack.
- Primary Substrates: Primary substrates are favored due to steric hindrance. Tertiary substrates are essentially unreactive via SN2 because the nucleophile cannot access the carbon atom bearing the leaving group.
- Aprotic Solvents: Aprotic solvents (like acetone or DMSO) don't hinder the nucleophile and stabilize the transition state.
Predicting the Major Product in SN2 Reactions
In SN2 reactions, the major product is easily predicted because the reaction occurs in a single step. The nucleophile replaces the leaving group with inversion of stereochemistry. No carbocation intermediate is formed, and therefore no rearrangements are possible.
Comparing SN1 and SN2 Reactions: A Summary Table
| Feature | SN1 | SN2 |
|---|---|---|
| Mechanism | Two-step | One-step, concerted |
| Rate-determining step | Carbocation formation | Nucleophilic attack |
| Kinetics | First-order (rate depends only on substrate concentration) | Second-order (rate depends on substrate and nucleophile concentrations) |
| Substrate | Tertiary > Secondary > Primary | Primary > Secondary (Tertiary is very slow or doesn't occur) |
| Nucleophile | Weak | Strong |
| Solvent | Protic | Aprotic |
| Stereochemistry | Racemization (if chiral substrate) | Inversion of configuration (Walden inversion) |
| Rearrangements | Possible | Not possible |
Illustrative Examples
Let's consider some examples to further illustrate predicting the major products in substitution reactions:
Example 1: SN1 Reaction
Consider the reaction of tert-butyl bromide with methanol. This reaction proceeds via an SN1 mechanism because of the tertiary substrate and the weak nucleophile (methanol). The major product is tert-butyl methyl ether.
Example 2: SN2 Reaction
Consider the reaction of methyl bromide with sodium iodide in acetone. This reaction is an SN2 reaction due to the primary substrate, the strong nucleophile (iodide), and the aprotic solvent (acetone). The major product is methyl iodide, formed with inversion of configuration (if the methyl group was part of a chiral molecule).
Example 3: Competition between SN1 and SN2
The reaction of a secondary substrate with a given nucleophile and solvent might proceed through both SN1 and SN2 pathways. The relative rates of the two pathways determine which product is the major product. If the nucleophile is strong and the solvent is aprotic, SN2 will dominate. If the nucleophile is weak and the solvent is protic, SN1 will dominate. If both mechanisms compete, a mixture of products will form with the major product being dictated by the kinetics of the two pathways.
Advanced Considerations: Steric Hindrance and Leaving Group Ability
The leaving group's ability to depart also plays a significant role. Better leaving groups (like tosylate or iodide) facilitate both SN1 and SN2 reactions. Steric hindrance around the reaction center affects the reaction rate. SN2 reactions are particularly sensitive to steric hindrance; bulky groups around the reaction center significantly slow down the reaction. SN1 reactions are less sensitive to steric effects because the leaving group departs before the nucleophile attacks.
Conclusion: Mastering Substitution Reactions
Predicting the major product of a substitution reaction requires a thorough understanding of both SN1 and SN2 mechanisms, the factors influencing each, and the interplay between them. By carefully considering the substrate, nucleophile, solvent, and leaving group, you can confidently determine the likely outcome of these crucial organic reactions. Remember that the stability of carbocations is central to SN1 reactions, while steric factors and the strength of the nucleophile are key to SN2 reactions. This detailed analysis provides the foundation for understanding and predicting the results of substitution reactions in a wide variety of synthetic scenarios. Through practice and application, you will master the art of predicting the major product of these reactions, a cornerstone of organic chemistry.
Latest Posts
Latest Posts
-
What Is The Difference Between Condensation And Accretion
Mar 27, 2025
-
Attorney Client Privilege May Ethically Be Revoked If
Mar 27, 2025
-
Good Strategy And Good Strategy Execution
Mar 27, 2025
-
For The Circuit Shown In The Figure
Mar 27, 2025
-
Acceleration Due To Gravity Of Jupiter
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Product Of The Substitution Reaction Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
