Draw The Major Product For The Dehydration Of 2 Pentanol
Holbox
Mar 16, 2025 · 5 min read
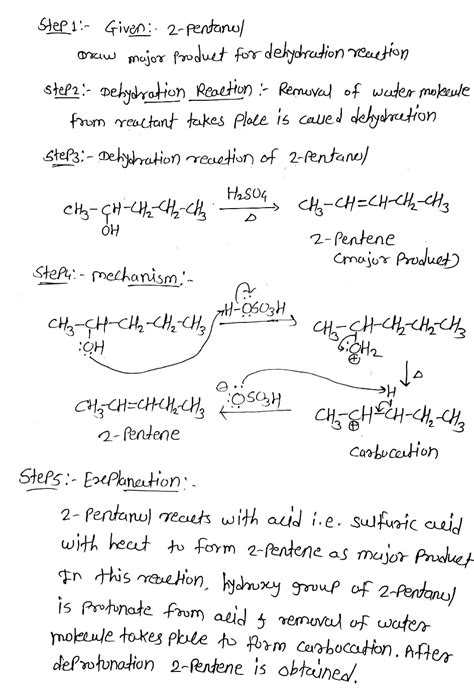
Table of Contents
- Draw The Major Product For The Dehydration Of 2 Pentanol
- Table of Contents
- Dehydration of 2-Pentanol: Predicting the Major Product
- Understanding the Dehydration Reaction
- The Mechanism: A Step-by-Step Breakdown
- Predicting the Major Product for 2-Pentanol Dehydration
- Zaitsev's Rule: The Guiding Principle
- Applying Zaitsev's Rule to 2-Pentanol
- Factors Influencing Product Distribution
- Detailed Analysis of the Major Product: 2-Pentene
- Experimental Considerations
- Conclusion: A Recap of 2-Pentanol Dehydration
- Latest Posts
- Latest Posts
- Related Post
Dehydration of 2-Pentanol: Predicting the Major Product
The dehydration of alcohols is a fundamental organic chemistry reaction, and understanding the factors influencing the product distribution is crucial for synthetic planning. This article delves into the dehydration of 2-pentanol, focusing on predicting the major product formed and exploring the underlying mechanisms and influencing factors.
Understanding the Dehydration Reaction
Alcohol dehydration is an elimination reaction where a molecule of water is removed from an alcohol, resulting in the formation of an alkene. This reaction typically requires an acidic catalyst, such as sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄), to protonate the hydroxyl group (-OH) and facilitate the departure of a water molecule. The mechanism involves the formation of a carbocation intermediate, which is crucial in determining the final product.
The Mechanism: A Step-by-Step Breakdown
-
Protonation: The acidic catalyst protonates the hydroxyl group of 2-pentanol, converting it into a better leaving group (water). This forms a protonated alcohol.
-
Carbocation Formation: The protonated alcohol loses a water molecule, generating a carbocation intermediate. This step is the rate-determining step of the reaction. The location of the positive charge on the carbocation is critical in determining the subsequent steps and ultimately, the product.
-
Hydride or Alkyl Shift (Optional): If a more stable carbocation can be formed through a hydride or alkyl shift (migration of a hydrogen or alkyl group), this will occur. This rearrangement leads to a more substituted carbocation, increasing stability.
-
Deprotonation: A base (often the conjugate base of the acid catalyst) abstracts a proton from a carbon adjacent to the carbocation. This results in the formation of a double bond (alkene) and the regeneration of the acid catalyst.
Predicting the Major Product for 2-Pentanol Dehydration
2-Pentanol is a secondary alcohol, meaning the hydroxyl group is attached to a secondary carbon atom. Upon dehydration, it can potentially yield two alkenes: 2-pentene and 1-pentene. However, one will be the major product due to Zaitsev's Rule.
Zaitsev's Rule: The Guiding Principle
Zaitsev's rule states that in an elimination reaction, the most substituted alkene (the alkene with the most alkyl groups attached to the double bond) is the major product. This is because more substituted alkenes are more stable due to hyperconjugation – the interaction between the electrons in the C-H sigma bonds and the empty p-orbital of the double bond. The increased stability of the more substituted alkene translates into a faster rate of formation.
Applying Zaitsev's Rule to 2-Pentanol
When 2-pentanol undergoes dehydration, the carbocation intermediate formed can undergo deprotonation at two different positions, leading to two possible alkenes:
-
2-pentene: Deprotonation from a carbon adjacent to the carbocation leads to 2-pentene. This alkene is more substituted and therefore more stable according to Zaitsev's rule. It is a disubstituted alkene.
-
1-pentene: Deprotonation from the terminal carbon leads to 1-pentene. This is a monosubstituted alkene, and thus less stable.
Therefore, based on Zaitsev's rule, 2-pentene is the major product of the dehydration of 2-pentanol.
Factors Influencing Product Distribution
While Zaitsev's rule provides a good prediction, other factors can subtly influence the product distribution:
-
Reaction Conditions: The temperature, concentration of the acid catalyst, and the solvent used can affect the reaction rate and product ratio. Higher temperatures generally favor the more substituted alkene (Zaitsev product).
-
Steric Hindrance: Bulky groups around the carbocation can hinder the approach of the base, potentially affecting the regioselectivity (the preference for formation of one isomer over another).
-
Carbocation Rearrangements: If a more stable carbocation can be formed through a hydride or alkyl shift, the reaction will proceed through this more stable intermediate, leading to a different product than initially predicted. This can override Zaitsev's rule in certain cases.
Detailed Analysis of the Major Product: 2-Pentene
2-Pentene exists as two geometric isomers: cis-2-pentene and trans-2-pentene. The trans isomer is generally more stable than the cis isomer due to reduced steric interactions between the alkyl groups. The dehydration of 2-pentanol will likely produce a mixture of both cis and trans-2-pentene, with the trans isomer being the major component. The exact ratio of cis to trans will depend on the reaction conditions.
Experimental Considerations
The actual experimental yield of 2-pentene might not perfectly match the theoretical prediction due to several reasons:
- Side Reactions: Other side reactions, such as polymerization of the alkene, can occur, reducing the yield of the desired product.
- Incomplete Reaction: The reaction might not go to completion, leaving unreacted 2-pentanol.
- Purification Challenges: Separating the different isomers of 2-pentene can be challenging.
Careful control of reaction conditions is essential to optimize the yield and selectivity of the reaction.
Conclusion: A Recap of 2-Pentanol Dehydration
The dehydration of 2-pentanol is a classic example of an elimination reaction governed by Zaitsev's rule. The major product is 2-pentene, a more substituted and thus more stable alkene, formed via a carbocation intermediate. Understanding the mechanism, Zaitsev's rule, and the influence of various factors is crucial for accurately predicting the outcome of this reaction and other similar elimination reactions. While the reaction predominantly produces 2-pentene, variations in reaction conditions and potential carbocation rearrangements can influence the exact product distribution and isomer ratios. Therefore, a comprehensive understanding of the reaction mechanism and influencing factors is essential for accurate predictions and successful experimental outcomes. Further experimentation and analysis would be needed for a complete understanding of the specific reaction conditions and yields obtained.
Latest Posts
Latest Posts
-
Which Of The Following Is True About The Function Below
Mar 17, 2025
-
Recall That In Cellular Respiration The Processes Of Glycolysis
Mar 17, 2025
-
One Main Issue In Studying Global Social Inequality Is
Mar 17, 2025
-
A Phrase Expressing The Aim Of A Group Or Party
Mar 17, 2025
-
Stakeholder Blank Is In Direct Contrast With Zero Sum Thinking
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Product For The Dehydration Of 2 Pentanol . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
