Draw The Major Organic Product X For The Below Reaction
Holbox
Mar 23, 2025 · 6 min read
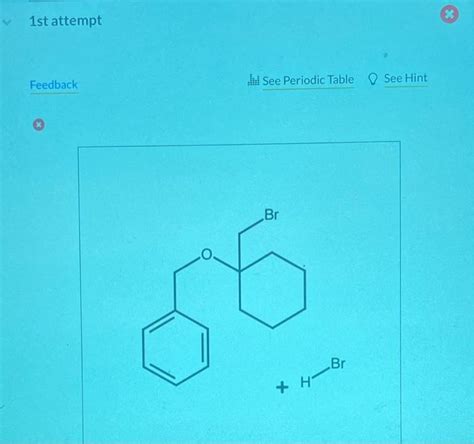
Table of Contents
- Draw The Major Organic Product X For The Below Reaction
- Table of Contents
- Drawing the Major Organic Product X: A Comprehensive Guide to Reaction Mechanisms and Prediction
- Understanding Reaction Mechanisms: The Foundation of Product Prediction
- 1. Nucleophilic Attack and Electrophilic Attack
- 2. Leaving Groups
- 3. Carbocation Stability
- 4. Transition States
- 5. Stereochemistry
- Predicting Regioselectivity and Stereoselectivity
- 1. Markovnikov's Rule
- 2. Anti-Markovnikov Addition
- 3. Steric Hindrance
- 4. Chelation
- Applying This Knowledge to Predict Product X
- Advanced Considerations
- Conclusion: A Systematic Approach to Product Prediction
- Latest Posts
- Related Post
Drawing the Major Organic Product X: A Comprehensive Guide to Reaction Mechanisms and Prediction
Predicting the major organic product of a reaction is a cornerstone of organic chemistry. Understanding reaction mechanisms allows us to not only identify the product but also to predict its stereochemistry and understand the factors influencing its formation. This article will delve into the process of determining the major organic product, focusing on a systematic approach that incorporates reaction mechanisms, regioselectivity, stereoselectivity, and other crucial factors. We won't be able to address every possible reaction, as that would be an impossible task; instead, we'll focus on building the foundational understanding needed to approach a wide range of scenarios. Note: Specific reactions will require specific knowledge, including functional group transformations and reagent reactivity.
Understanding Reaction Mechanisms: The Foundation of Product Prediction
Before attempting to predict the product of any reaction, a solid grasp of the underlying mechanism is crucial. A reaction mechanism outlines the step-by-step process by which reactants are converted into products. Key concepts to master include:
1. Nucleophilic Attack and Electrophilic Attack
Many organic reactions involve nucleophiles (electron-rich species) attacking electrophiles (electron-deficient species). Understanding which atom or functional group acts as the nucleophile and which acts as the electrophile is paramount in predicting product formation.
- Examples of Nucleophiles: Alkoxides (RO-), amines (R3N), Grignard reagents (RMgX), carbanions.
- Examples of Electrophiles: Carbonyls (C=O), alkyl halides (RX), carbocations.
2. Leaving Groups
In many substitution and elimination reactions, a leaving group departs from a molecule, creating a reactive site for nucleophilic or electrophilic attack. Good leaving groups are typically weak bases, such as halides (Cl-, Br-, I-), tosylates (OTs), and mesylates (OMs).
3. Carbocation Stability
Carbocation intermediates are frequently involved in reactions like SN1 and E1. The stability of a carbocation significantly influences the regioselectivity and stereoselectivity of the reaction. Tertiary carbocations are the most stable, followed by secondary, then primary, with methyl carbocations being the least stable. Resonance stabilization further enhances carbocation stability.
4. Transition States
Transition states represent the highest-energy point along the reaction coordinate. Understanding the structure and energy of the transition state can provide insights into the reaction's rate and stereochemistry.
5. Stereochemistry
Stereochemistry plays a vital role in determining the structure of the product. Consider factors like chirality, enantioselectivity, and diastereoselectivity. Reactions can proceed with retention, inversion, or racemization of stereochemistry, depending on the mechanism.
Predicting Regioselectivity and Stereoselectivity
Regioselectivity refers to the preferential formation of one regioisomer over another, while stereoselectivity refers to the preferential formation of one stereoisomer over another. Several factors influence these aspects:
1. Markovnikov's Rule
In electrophilic addition reactions to alkenes, Markovnikov's rule predicts that the electrophile (often a proton) will add to the carbon atom with the greater number of hydrogen atoms, while the nucleophile adds to the other carbon. This is due to the formation of a more stable carbocation intermediate.
2. Anti-Markovnikov Addition
In some cases, anti-Markovnikov addition can occur, typically involving free radical mechanisms. For example, the addition of HBr to alkenes in the presence of peroxides leads to anti-Markovnikov addition.
3. Steric Hindrance
Steric hindrance, the repulsion between bulky groups, can influence the regioselectivity and stereoselectivity of a reaction. Reactions often favor pathways that minimize steric interactions.
4. Chelation
Chelation, the formation of a ring-like structure involving a metal ion, can influence the regio- and stereoselectivity of reactions.
Applying This Knowledge to Predict Product X
Let's consider some examples. To illustrate, we will need a specific reaction. Since none was provided, I will create hypothetical examples showcasing different reaction types and considerations. Remember, the crucial step is to identify the mechanism and then apply the principles of regioselectivity and stereoselectivity.
Example 1: SN1 Reaction
Imagine a reaction involving a tertiary alkyl halide (like tert-butyl bromide) reacting with methanol. The mechanism is SN1:
- Ionization: The alkyl halide ionizes to form a carbocation and a bromide ion. The tertiary carbocation is relatively stable.
- Nucleophilic Attack: Methanol, acting as a nucleophile, attacks the carbocation, forming a new C-O bond.
- Proton Transfer: A proton transfer completes the reaction, resulting in the formation of tert-butyl methyl ether.
In this case, the major product X is tert-butyl methyl ether. Because the carbocation intermediate is planar, the attack by methanol can occur from either face, leading to a racemic mixture.
Example 2: SN2 Reaction
Consider the reaction between methyl iodide and sodium cyanide (NaCN). This is an SN2 reaction:
- Nucleophilic Attack: Cyanide ion (CN-) acts as a nucleophile and attacks the methyl carbon from the backside. This leads to inversion of configuration.
- Leaving Group Departure: Iodide ion (I-) departs.
- Product Formation: The major product X is acetonitrile (CH3CN). The reaction proceeds with inversion of configuration, meaning the stereochemistry at the reaction center is inverted.
Example 3: Electrophilic Addition to Alkenes
Consider the addition of HBr to propene. This follows Markovnikov's rule:
- Protonation: The proton (H+) adds to the less substituted carbon (the terminal carbon) to form a secondary carbocation intermediate.
- Nucleophilic Attack: The bromide ion (Br-) acts as the nucleophile and attacks the carbocation.
- Product Formation: The major product X is 2-bromopropane. The bromine atom is added to the more substituted carbon, following Markovnikov's rule.
Example 4: Elimination Reactions
Consider the dehydration of 2-butanol. This is an E1 reaction:
- Protonation: The hydroxyl group is protonated to form a good leaving group (water).
- Leaving Group Departure: Water leaves, forming a secondary carbocation.
- Deprotonation: A base abstracts a proton from a carbon adjacent to the carbocation, forming a double bond. The more substituted alkene (2-butene) is the major product due to greater stability.
The major product X in this case is 2-butene (the more substituted alkene is favored).
Advanced Considerations
Many reactions are not as straightforward as the examples above. Advanced considerations include:
- Competing Reactions: Multiple reactions might occur simultaneously. Determining the major product requires assessing the relative rates of the competing reactions.
- Catalyst Effects: Catalysts can significantly alter the reaction pathway, leading to different products.
- Solvent Effects: The choice of solvent can influence the reaction mechanism and product distribution.
- Temperature and Pressure: Reaction conditions (temperature and pressure) can affect the equilibrium and kinetics of the reaction.
Conclusion: A Systematic Approach to Product Prediction
Predicting the major organic product X requires a systematic approach:
- Identify the functional groups and reagents.
- Determine the likely reaction mechanism.
- Consider regioselectivity and stereoselectivity based on the mechanism.
- Evaluate factors like carbocation stability, steric hindrance, and leaving group ability.
- Consider competing reactions and the effects of reaction conditions.
By mastering these principles and practicing with various examples, you can confidently predict the major organic products of a wide range of reactions. Remember that this is a skill that improves with practice and a deep understanding of organic chemistry principles. Continue to explore different reaction types and mechanisms to solidify your understanding and ability to predict the major product in complex scenarios.
Latest Posts
Related Post
Thank you for visiting our website which covers about Draw The Major Organic Product X For The Below Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.