Draw The Major Organic Product S Of The Following Reaction
Holbox
Mar 24, 2025 · 6 min read
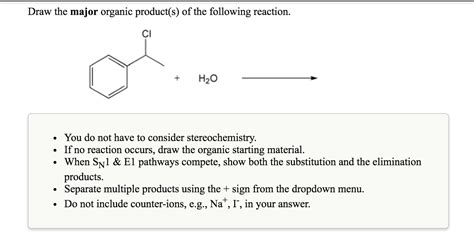
Table of Contents
- Draw The Major Organic Product S Of The Following Reaction
- Table of Contents
- Drawing the Major Organic Products of Reactions: A Comprehensive Guide
- Understanding Reaction Mechanisms: The Key to Predicting Products
- Common Reaction Types and Product Prediction
- 1. SN1 and SN2 Reactions
- 2. E1 and E2 Reactions
- 3. Addition Reactions
- 4. Oxidation and Reduction Reactions
- Factors Influencing Product Distribution
- A Systematic Approach to Predicting Products
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Major Organic Products of Reactions: A Comprehensive Guide
Predicting the major organic product of a reaction is a cornerstone of organic chemistry. This skill requires a deep understanding of reaction mechanisms, functional group transformations, and the principles that govern regio- and stereoselectivity. This article will delve into various reaction types, exploring the factors that influence product formation and providing a systematic approach to accurately predict the major organic product. We'll cover a range of reactions, emphasizing the reasoning behind product selection and the application of fundamental organic chemistry principles.
Understanding Reaction Mechanisms: The Key to Predicting Products
Before we delve into specific reactions, it's crucial to grasp the concept of reaction mechanisms. A reaction mechanism is a step-by-step description of how a reaction proceeds, detailing the movement of electrons and the formation and breaking of bonds. Understanding the mechanism allows us to predict the intermediate structures formed during the reaction, ultimately guiding us towards the final product.
Different reaction mechanisms have distinct characteristics which heavily influence the final product obtained. Some key mechanistic considerations include:
- Nucleophilic attack: Nucleophiles, electron-rich species, attack electrophilic centers, electron-deficient regions of a molecule. The outcome often depends on the nucleophile's strength, the electrophile's reactivity, and steric hindrance.
- Electrophilic attack: Electrophiles, electron-deficient species, attack nucleophilic sites. Similarly, the electrophile's strength and the nucleophile's accessibility influence the outcome.
- Carbocation rearrangements: Carbocations, positively charged carbon intermediates, are prone to rearrangements (hydride or alkyl shifts) to achieve greater stability. This rearrangement often dictates the final product structure.
- Stereochemistry: The three-dimensional arrangement of atoms in a molecule significantly impacts reactivity. Understanding stereochemistry is critical for predicting stereoselective reactions, producing specific stereoisomers (enantiomers or diastereomers).
Common Reaction Types and Product Prediction
Let's examine several common reaction types and illustrate how to determine the major organic products.
1. SN1 and SN2 Reactions
These nucleophilic substitution reactions differ significantly in their mechanisms and consequently in their product distributions.
- SN1 (Substitution Nucleophilic Unimolecular): This two-step mechanism involves a carbocation intermediate. The rate-determining step is the ionization of the substrate to form the carbocation, making the reaction unimolecular (first-order kinetics). Carbocation rearrangements are common, leading to different products than expected based on direct substitution. SN1 reactions typically favor racemization at the reaction center.
Example: The reaction of tert-butyl bromide with methanol will predominantly yield tert-butyl methyl ether through an SN1 mechanism. The tertiary carbocation intermediate is relatively stable.
- SN2 (Substitution Nucleophilic Bimolecular): This one-step concerted mechanism involves simultaneous bond breaking and bond formation. The reaction rate depends on both the substrate and the nucleophile concentration (second-order kinetics). SN2 reactions typically lead to inversion of configuration at the reaction center (Walden inversion). Stronger nucleophiles favor SN2 reactions, and steric hindrance in the substrate significantly reduces the reaction rate.
Example: The reaction of methyl bromide with hydroxide ion will primarily yield methanol via an SN2 mechanism. The methyl group experiences minimal steric hindrance.
2. E1 and E2 Reactions
These elimination reactions remove a leaving group and a proton from adjacent carbons, forming an alkene.
- E1 (Elimination Unimolecular): Similar to SN1, E1 reactions proceed via a carbocation intermediate. The rate-determining step is the formation of the carbocation, and rearrangements are possible. E1 reactions often compete with SN1 reactions under the same conditions. The product distribution is determined by Zaitsev's rule (favoring the most substituted alkene).
Example: Dehydration of 2-methyl-2-propanol (tert-butyl alcohol) using a strong acid like sulfuric acid will primarily yield 2-methylpropene (isobutene) via an E1 mechanism.
- E2 (Elimination Bimolecular): E2 reactions are concerted, involving simultaneous removal of a proton and a leaving group. The stereochemistry of the substrate significantly impacts the product. Anti-periplanar geometry is preferred (leaving group and proton are on opposite sides of the molecule). Zaitsev's rule also applies here. Strong bases favor E2 reactions.
Example: Dehydrohalogenation of 2-bromobutane with a strong base like potassium tert-butoxide will primarily yield 2-butene (the more substituted alkene) via an E2 mechanism.
3. Addition Reactions
Addition reactions involve the addition of atoms or groups to a multiple bond (C=C or C≡C). The regioselectivity and stereoselectivity of addition reactions are crucial in determining the product.
- Electrophilic Addition: Electrophiles add to alkenes, forming carbocation intermediates. Markovnikov's rule dictates the regioselectivity, where the electrophile adds to the carbon with fewer alkyl substituents. Stereochemistry can be syn (addition from the same side) or anti (addition from opposite sides), depending on the reaction mechanism.
Example: The addition of HBr to propene will yield 2-bromopropane, following Markovnikov's rule.
- Nucleophilic Addition: Nucleophiles attack carbonyl compounds (aldehydes and ketones), forming tetrahedral intermediates. The final product depends on the nature of the nucleophile and the reaction conditions.
Example: The reaction of formaldehyde with a Grignard reagent (organomagnesium halide) will yield a primary alcohol after workup.
4. Oxidation and Reduction Reactions
These reactions involve changes in the oxidation state of carbon atoms.
- Oxidation: Increases the oxidation state of carbon. Common oxidizing agents include potassium permanganate (KMnO4) and chromic acid (H2CrO4). The products depend on the substrate and the oxidizing agent's strength.
Example: Oxidation of a primary alcohol with chromic acid typically yields a carboxylic acid.
- Reduction: Decreases the oxidation state of carbon. Common reducing agents include lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4).
Example: Reduction of a ketone with sodium borohydride typically yields a secondary alcohol.
Factors Influencing Product Distribution
Several factors beyond the basic mechanism influence the major product obtained:
- Steric hindrance: Bulky groups can hinder nucleophilic or electrophilic attack, affecting reaction rates and regioselectivity.
- Temperature: Higher temperatures often favor elimination reactions over substitution reactions.
- Solvent: Polar protic solvents favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 and E2 reactions.
- Catalyst: Catalysts can significantly alter reaction pathways and product selectivity.
A Systematic Approach to Predicting Products
To confidently predict the major organic product, follow a systematic approach:
- Identify the functional groups: Determine the reactive functional groups present in the reactants.
- Identify the reaction type: Determine the type of reaction based on the reactants and reaction conditions.
- Draw the mechanism: Draw a detailed step-by-step mechanism, including intermediates.
- Consider regio- and stereoselectivity: Predict the regiochemistry and stereochemistry of the product based on the mechanism and reaction conditions.
- Consider competing reactions: Identify and evaluate any competing reactions that might affect product distribution.
- Predict the major product: Based on the mechanism, reaction conditions, and the considerations above, predict the major organic product.
Conclusion
Predicting the major organic product of a reaction is a challenging but crucial skill in organic chemistry. By mastering reaction mechanisms, understanding the factors that influence product distribution, and adopting a systematic approach, you can confidently predict the outcome of a wide range of organic reactions. Remember that practice is key; the more reactions you analyze and predict, the more proficient you will become. This detailed exploration of various reaction types and influencing factors should provide a strong foundation for successfully tackling organic chemistry problems. Continuous study and practice will solidify your understanding and improve your predictive capabilities.
Latest Posts
Latest Posts
-
Air At 30000 Feet Is At A Temperature Of
Mar 29, 2025
-
Performance Evaluations Are Best Done Using
Mar 29, 2025
-
Silver Company Makes A Product That Is Very Popular
Mar 29, 2025
-
Dana Works At The Hospital And Interacts With A Patient
Mar 29, 2025
-
All Of The Following Are Positive Aspects Of Aging Except
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Organic Product S Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
