Draw The Major Monobromination Product Of This Reaction
Holbox
Mar 18, 2025 · 5 min read
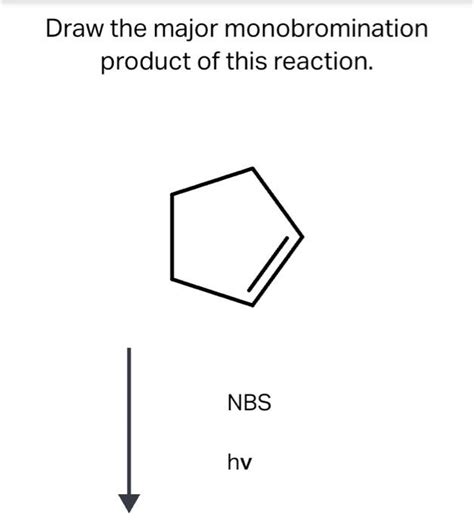
Table of Contents
Draw the Major Monobromination Product of This Reaction: A Comprehensive Guide
Understanding organic chemistry reactions, particularly electrophilic aromatic substitution, is crucial for success in chemistry. This article delves into predicting the major monobromination product in various reactions, focusing on the principles of regioselectivity and the directing effects of substituents. We'll explore the mechanisms behind these reactions and provide a step-by-step approach to accurately determining the major product.
Understanding Electrophilic Aromatic Substitution (EAS)
Electrophilic aromatic substitution is a fundamental reaction type in organic chemistry where an electrophile replaces a hydrogen atom on an aromatic ring. Bromination, a specific type of EAS, involves the addition of a bromine atom to the aromatic ring. The reaction typically utilizes a Lewis acid catalyst, such as iron(III) bromide (FeBr₃) or aluminum bromide (AlBr₃), to generate a more electrophilic bromine species.
The Mechanism of Bromination
The bromination mechanism proceeds through several key steps:
-
Generation of the Electrophile: The catalyst, like FeBr₃, reacts with bromine (Br₂) to form a complex that polarizes the bromine molecule, making one bromine atom more electrophilic. This electrophile is often represented as Br⁺, though it's more accurately a complex.
-
Electrophilic Attack: The electrophile attacks the electron-rich aromatic ring, forming a resonance-stabilized carbocation intermediate (also called a sigma complex or arenium ion). This step is the rate-determining step.
-
Deprotonation: A base (often a bromide ion, Br⁻) abstracts a proton from the carbocation, restoring the aromaticity of the ring and forming the brominated product.
Regioselectivity: Predicting the Major Product
The key to correctly drawing the major monobromination product lies in understanding regioselectivity – the preference for substitution at one position over another on the aromatic ring. This is largely governed by the directing effects of substituents already present on the ring.
Directing Effects of Substituents
Substituents on the benzene ring can be broadly classified into two categories based on their directing effects:
1. Ortho/Para-Directing Groups: These groups activate the ring towards electrophilic aromatic substitution and direct the incoming electrophile to the ortho (adjacent) and para (opposite) positions. Common ortho/para directing groups include:
- Alkyl groups (-CH₃, -C₂H₅, etc.): These groups are weakly activating and ortho/para directing due to their electron-donating inductive effect.
- Hydroxyl groups (-OH): Strongly activating and ortho/para directing due to resonance and inductive effects.
- Amino groups (-NH₂): Strongly activating and ortho/para directing due to strong resonance effects.
- Methoxy groups (-OCH₃): Strongly activating and ortho/para directing due to resonance effects.
2. Meta-Directing Groups: These groups deactivate the ring towards electrophilic aromatic substitution and direct the incoming electrophile to the meta (1,3) position. Common meta-directing groups include:
- Nitro groups (-NO₂): Strongly deactivating and meta directing due to strong electron-withdrawing inductive and resonance effects.
- Carboxylic acid groups (-COOH): Deactivating and meta directing.
- Cyano groups (-CN): Deactivating and meta directing.
- Sulfonic acid groups (-SO₃H): Deactivating and meta directing.
Steric Hindrance
While directing effects guide the preference for substitution, steric hindrance can sometimes play a significant role. If a bulky ortho/para directing group is present, the para position might be favored even if the ortho position is also activated, simply because the bulky group hinders the approach of the electrophile at the ortho position.
Step-by-Step Approach to Predicting the Major Monobromination Product
Let's consider several examples to illustrate how to determine the major monobromination product:
Example 1: Bromination of Toluene
Toluene (methylbenzene) has a methyl group (-CH₃), which is an ortho/para-directing group. Therefore, bromination will predominantly occur at the ortho and para positions. However, due to less steric hindrance, the para product will be the major product.
(Image: Show the structure of toluene, then the structure of the para-bromotoluene as the major product)
Example 2: Bromination of Nitrobenzene
Nitrobenzene has a nitro group (-NO₂), a meta-directing group. Bromination will occur primarily at the meta position.
(Image: Show the structure of nitrobenzene, then the structure of meta-bromonitrobenzene as the major product)
Example 3: Bromination of Phenol
Phenol has a hydroxyl group (-OH), a strongly activating and ortho/para-directing group. Both ortho and para positions are activated, but steric hindrance might favor the para product as major. However, in some cases, the ortho product may still be favored.
(Image: Show the structure of phenol. Then show both ortho-bromophenol and para-bromophenol, indicating para-bromophenol as the major but noting that ortho can also be significantly produced.)
Example 4: Bromination of Aniline
Aniline has an amino group (-NH₂), a strongly activating and ortho/para-directing group. Similar to phenol, both ortho and para positions are activated, with steric considerations influencing the major product. However, aniline reacts vigorously, and protection of the amino group is often necessary to control the reaction.
(Image: Show the structure of aniline. Then show both ortho-bromoaniline and para-bromoaniline, indicating a similar ratio and emphasizing that conditions can significantly influence the major product.)
Example 5: Bromination of m-Xylene
m-Xylene (1,3-dimethylbenzene) presents a more complex scenario. Both methyl groups are ortho/para-directing. The substitution will happen at one of the positions that is ortho to one methyl group and para to the other.
(Image: Show the structure of m-xylene, then show the two possible monobromination products, highlighting the major product which would be determined by steric hindrance considerations and the relative rate of attack at those positions.)
Beyond Monobromination: Considering Multiple Substituents and Further Reactions
When multiple substituents are present, the overall directing effect is a combination of the individual directing effects. Predicting the major product requires careful consideration of all substituents and their relative strengths. Moreover, the reaction conditions (temperature, solvent, etc.) can also influence the regioselectivity.
After monobromination, further reactions are possible depending on the substituents present. For example, the brominated product might undergo further electrophilic aromatic substitutions, nucleophilic aromatic substitutions, or other reactions specific to the functional groups.
Conclusion
Predicting the major monobromination product requires a solid understanding of electrophilic aromatic substitution, regioselectivity, and the directing effects of various substituents. By systematically analyzing the substituents and considering steric factors, you can accurately determine the major product in most monobromination reactions. Remember, practice is key to mastering this important aspect of organic chemistry. Working through numerous examples will help you become proficient in predicting the outcome of these reactions. This article serves as a comprehensive foundation; further exploration of advanced topics like activating/deactivating strengths and the effects of specific reaction conditions will enhance your understanding even further.
Latest Posts
Latest Posts
-
What Is Unique About The Highlighted Veins
Mar 18, 2025
-
Which Of The Following Is Mismatched
Mar 18, 2025
-
The Term For Pertaining To The Sun Is
Mar 18, 2025
-
Jack And Jill Exercise In A 25 0 M Long Swimming Pool
Mar 18, 2025
-
Which Of The Following Is True About Corporations
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Monobromination Product Of This Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
