Draw The Major And Minor Monobromination Products Of This Reaction
Holbox
Mar 20, 2025 · 5 min read
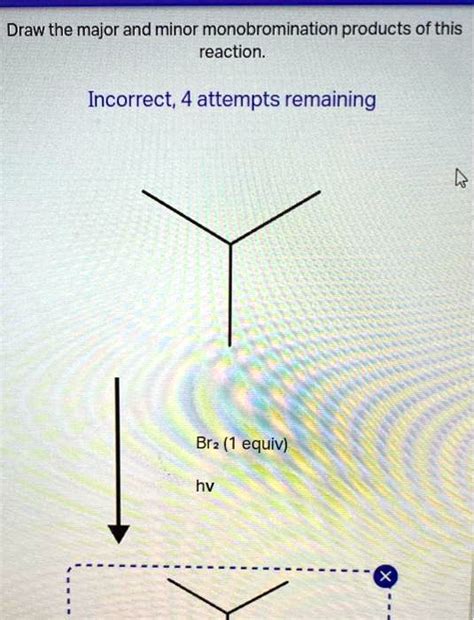
Table of Contents
Predicting Major and Minor Monobromination Products: A Deep Dive into Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (EAS) is a fundamental reaction in organic chemistry, enabling the introduction of various electrophiles onto aromatic rings. Understanding the regioselectivity—the preference for substitution at a particular position—is crucial for predicting the products of these reactions. This article will delve into the monobromination of an unspecified aromatic compound, exploring the factors that determine the major and minor products formed. While a specific compound isn't provided, we can explore the general principles and apply them to various scenarios.
Understanding Electrophilic Aromatic Substitution (EAS)
Before predicting the products of monobromination, let's refresh our understanding of the EAS mechanism. The reaction involves the following key steps:
1. Formation of the Electrophile:
The electrophile, in this case, is a bromine cation (Br+), which is generated through the reaction of bromine (Br2) with a Lewis acid catalyst, typically iron(III) bromide (FeBr3) or aluminum bromide (AlBr3). This catalyst polarizes the bromine molecule, making it more susceptible to nucleophilic attack by the aromatic ring.
2. Electrophilic Attack:
The aromatic ring, rich in π electrons, acts as a nucleophile, attacking the electrophilic bromine cation. This forms a resonance-stabilized carbocation intermediate, often referred to as a σ-complex or arenium ion. This intermediate is crucial in determining the regioselectivity of the reaction.
3. Deprotonation:
A base (often a bromide ion, Br-) abstracts a proton from the carbocation, restoring the aromaticity of the ring and forming the monobrominated product.
Factors Influencing Regioselectivity in Monobromination
Several factors dictate where the bromine atom will preferentially substitute on the aromatic ring. The most significant are:
1. The Effect of Existing Substituents:
If the aromatic ring already possesses substituents, these groups exert a profound influence on the regioselectivity of further substitution. Substituents are categorized as either activating or deactivating, and as ortho/para-directing or meta-directing.
Activating, Ortho/Para-Directing Groups: These groups donate electron density to the ring, making it more reactive towards electrophilic attack and favoring substitution at the ortho (adjacent) and para (opposite) positions. Examples include:
- -OH (hydroxyl): Strongly activating and ortho/para-directing.
- -NH2 (amino): Strongly activating and ortho/para-directing.
- -OCH3 (methoxy): Strongly activating and ortho/para-directing.
- -CH3 (methyl): Weakly activating and ortho/para-directing.
Deactivating, Meta-Directing Groups: These groups withdraw electron density from the ring, making it less reactive towards electrophilic attack and favoring substitution at the meta position. Examples include:
- -NO2 (nitro): Strongly deactivating and meta-directing.
- -COOH (carboxyl): Moderately deactivating and meta-directing.
- -SO3H (sulfonic acid): Moderately deactivating and meta-directing.
- -CN (cyano): Strongly deactivating and meta-directing.
Deactivating, Ortho/Para-Directing Groups: A small number of groups are deactivating but still direct ortho/para. These are usually halogens.
- -F, -Cl, -Br, -I (halogens): Deactivating but ortho/para-directing. The deactivation is due to the electronegativity of the halogen, which withdraws electron density. However, the lone pairs on the halogen can donate electron density through resonance, making ortho/para substitution slightly favorable.
2. Steric Hindrance:
Even when a substituent is ortho/para-directing, steric hindrance can play a significant role. If the ortho positions are blocked by bulky substituents, substitution will predominantly occur at the para position.
3. Resonance Effects:
The resonance structures of the carbocation intermediate are crucial in determining regioselectivity. The stability of these intermediates is directly related to the position of substitution. More stable intermediates lead to higher yields of the corresponding product.
Examples of Monobromination: Predicting Major and Minor Products
Let's consider some examples to illustrate how to predict the major and minor products of monobromination:
Example 1: Monobromination of Toluene
Toluene (methylbenzene) has a methyl group (-CH3), which is weakly activating and ortho/para-directing. Therefore, we expect bromination to occur predominantly at the ortho and para positions. Due to slightly less steric hindrance, the para product is usually slightly more favored, although the ortho and para isomers are usually obtained as a mixture.
- Major Product: para-bromotoluene
- Minor Product: ortho-bromotoluene
Example 2: Monobromination of Nitrobenzene
Nitrobenzene has a nitro group (-NO2), which is strongly deactivating and meta-directing. Bromination will occur predominantly at the meta position.
- Major Product: meta-bromonitrobenzene
- Minor Products: ortho-bromonitrobenzene and para-bromonitrobenzene (significantly less favored)
Example 3: Monobromination of Phenol
Phenol has a hydroxyl group (-OH), which is strongly activating and ortho/para-directing. However, the ortho positions are more susceptible to steric hindrance from the bulky –OH group. Therefore, we might observe a higher proportion of the para product.
- Major Product: para-bromophenol
- Minor Product: ortho-bromophenol
Example 4: Monobromination of Chlorobenzene
Chlorobenzene presents a more nuanced case. Chlorine is deactivating but ortho/para-directing. The deactivating effect is more significant than the activating effect from the resonance, resulting in a slower reaction compared to benzene. Steric hindrance might also favor para substitution over ortho.
- Major Product: para-bromochlorobenzene
- Minor Product: ortho-bromochlorobenzene
Beyond Monobromination: Further Considerations
The principles outlined above apply to other electrophilic aromatic substitutions besides bromination. Reactions with other electrophiles, such as nitration, sulfonation, and Friedel-Crafts alkylation, follow similar regiochemical patterns.
Conclusion: Predicting Regioselectivity in EAS
Predicting the major and minor products of electrophilic aromatic substitution requires a thorough understanding of the electronic effects of substituents, steric hindrance, and the resonance stabilization of the carbocation intermediates. By carefully considering these factors, organic chemists can successfully design syntheses and predict the outcome of EAS reactions. Remember that while we can predict the major product, the minor products are often formed, and their proportion depends on many factors and the conditions of the reaction. Further analysis, such as using techniques such as Gas Chromatography-Mass Spectrometry (GC-MS) or Nuclear Magnetic Resonance (NMR) spectroscopy, may be required to precisely quantify the product ratios obtained in any given reaction. This detailed understanding of reaction mechanisms is critical for effective organic synthesis.
Latest Posts
Latest Posts
-
Readings For Diversity And Social Justice 4th Edition
Mar 21, 2025
-
The Determination Of An Equilibrium Constant Lab Answers Vernier
Mar 21, 2025
-
The Term Discrimination Is Defined In The Text As
Mar 21, 2025
-
Focus Forecasting Is Based On The Principle That
Mar 21, 2025
-
Will Jill And Phil Are All Wheat Farmers
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major And Minor Monobromination Products Of This Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
