Draw The Fischer Projection Of The Four Aldotetroses
Holbox
Mar 30, 2025 · 5 min read
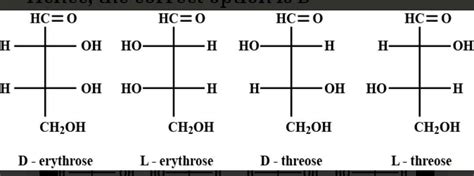
Table of Contents
- Draw The Fischer Projection Of The Four Aldotetroses
- Table of Contents
- Drawing the Fischer Projections of the Four Aldotetroses: A Comprehensive Guide
- Understanding Fischer Projections
- Identifying Chiral Centers in Aldotetroses
- The Four Aldotetroses: A Systematic Approach
- 1. D-Threose
- 2. L-Threose
- 3. D-Erythrose
- 4. L-Erythrose
- Diastereomers and Epimers: Exploring Relationships
- Importance of Fischer Projections in Carbohydrate Chemistry
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Fischer Projections of the Four Aldotetroses: A Comprehensive Guide
Aldotetroses are monosaccharides containing four carbon atoms and an aldehyde functional group. Understanding their Fischer projections is fundamental to grasping carbohydrate chemistry. This comprehensive guide will walk you through drawing the four possible aldotetroses, explaining the logic behind Fischer projections and highlighting key stereochemical features. We'll delve into the intricacies of chirality and explore how to systematically represent these molecules using this invaluable tool.
Understanding Fischer Projections
Fischer projections are a two-dimensional representation of three-dimensional molecules, particularly useful for depicting chiral centers in carbohydrates. They employ specific conventions:
- Vertical lines: Represent bonds projecting away from the viewer (into the page).
- Horizontal lines: Represent bonds projecting towards the viewer (out of the page).
- Intersection: The intersection represents a carbon atom (unless otherwise indicated).
The power of Fischer projections lies in their ability to clearly illustrate the stereochemistry—the spatial arrangement of atoms—around chiral centers. This is crucial for aldotetroses, as they possess multiple chiral centers.
Identifying Chiral Centers in Aldotetroses
Chirality is a property of a molecule that is not superimposable on its mirror image. A carbon atom is considered chiral if it's bonded to four different groups. Aldotetroses, with their aldehyde group, three carbon atoms, and various hydroxyl (-OH) groups, often possess multiple chiral centers.
Let's consider a general aldotetrose structure:
CHO
|
H-C-OH
|
H-C-OH
|
CH2OH
Notice that the second and third carbon atoms (C-2 and C-3) each have four different groups attached, making them chiral centers. The first carbon (C-1) is part of the aldehyde and is not chiral, and the last carbon (C-4) is bonded to three hydrogen atoms and one carbon atom, again not chiral. Therefore, an aldotetrose has two chiral centers.
The Four Aldotetroses: A Systematic Approach
Because each chiral center has two possible configurations (R or S, or more simply, D or L as we'll discuss), there are 2<sup>n</sup> possible stereoisomers, where 'n' is the number of chiral centers. For aldotetroses (n=2), this means there are 2<sup>2</sup> = 4 possible stereoisomers.
We can systematically derive these four aldotetroses using the Fischer projection method. We'll use the D/L system, a common nomenclature system for carbohydrates. In the D/L system, the configuration of the highest numbered chiral center is used to determine D or L designation. If the -OH group on the highest numbered chiral center (C-3 in this case) projects to the right, the aldotetrose is designated as D. If it projects to the left, it's designated as L.
Here’s how we can draw them:
1. D-Threose
CHO
|
H-C-OH (D configuration at C-3)
|
HO-C-H (This determines it is Threose, not Erythrose)
|
CH2OH
D-Threose is one of the four aldotetroses. Note the -OH group on C-3 is on the right, and the -OH group on C-2 is on the left, which, alongside the D configuration, uniquely identifies this specific aldotetrose.
2. L-Threose
CHO
|
H-C-OH (L configuration at C-3)
|
HO-C-H (This determines it is Threose, not Erythrose)
|
CH2OH
L-Threose is the enantiomer (mirror image) of D-Threose. The only difference is the configuration at C-3; the -OH group is now on the left.
3. D-Erythrose
CHO
|
H-C-OH (D configuration at C-3)
|
H-C-OH (This determines it is Erythrose, not Threose)
|
CH2OH
D-Erythrose differs from D-Threose only in the configuration at C-2. Notice that the -OH groups on both C-2 and C-3 are on the right (in the case of the D-isomer), giving a unique structural identity.
4. L-Erythrose
CHO
|
H-C-OH (L configuration at C-3)
|
H-C-OH (This determines it is Erythrose, not Threose)
|
CH2OH
L-Erythrose is the enantiomer of D-Erythrose. The configuration at C-3 has been inverted.
Diastereomers and Epimers: Exploring Relationships
The four aldotetroses are not just individual molecules; they have specific relationships with each other:
-
Enantiomers: D-Threose and L-Threose are enantiomers (mirror images). Similarly, D-Erythrose and L-Erythrose are enantiomers. Enantiomers have identical physical properties except for their interaction with plane-polarized light.
-
Diastereomers: D-Threose and D-Erythrose are diastereomers. Diastereomers are stereoisomers that are not mirror images. The same relationship exists between L-Threose and L-Erythrose. Diastereomers have different physical properties.
-
Epimers: D-Threose and D-Erythrose are also epimers. Epimers are a specific type of diastereomer that differ in the configuration at only one chiral center.
Importance of Fischer Projections in Carbohydrate Chemistry
Fischer projections are essential for:
-
Understanding stereochemistry: They visually depict the spatial arrangement of atoms, crucial for understanding the properties and reactions of carbohydrates.
-
Predicting reactions: The configuration at each chiral center influences the reactivity of the molecule. Fischer projections facilitate prediction of reaction outcomes.
-
Naming and classifying carbohydrates: The D/L system relies heavily on Fischer projections for unambiguous nomenclature.
-
Analyzing complex carbohydrates: While more complex carbohydrates have many more chiral centers, the fundamental principles established with aldotetroses remain vital for understanding their structure and properties.
Conclusion
Mastering the art of drawing Fischer projections for aldotetroses is a cornerstone of understanding carbohydrate chemistry. By systematically applying the conventions of Fischer projections and understanding the concepts of chirality, enantiomers, diastereomers, and epimers, you gain a strong foundation for tackling more complex carbohydrate structures and their reactions. This knowledge is critical in fields like biochemistry, medicine, and food science, where carbohydrates play an essential role. Remember to practice drawing these structures until you feel confident in your ability to represent the spatial arrangement of atoms accurately and efficiently. This skill will serve you well in your future studies of organic and biological chemistry.
Latest Posts
Latest Posts
-
Correctly Label The Components Of The Respiratory System
Apr 02, 2025
-
What Is The Electron Configuration For Aluminum
Apr 02, 2025
-
A Customer Is Calling Her Insurance Company
Apr 02, 2025
-
A Device Consisting Of Four Heavy Balls
Apr 02, 2025
-
You Receive An Email Marked Important From Your Boss
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Draw The Fischer Projection Of The Four Aldotetroses . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
