Draw The Electron Configuration For A Neutral Atom Of Cobalt
Holbox
Mar 20, 2025 · 5 min read
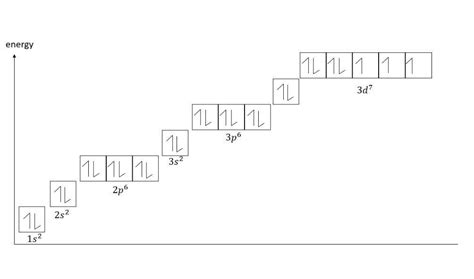
Table of Contents
Drawing the Electron Configuration for a Neutral Atom of Cobalt: A Comprehensive Guide
Cobalt, a transition metal with the symbol Co and atomic number 27, presents a fascinating case study in electron configuration. Understanding its electron configuration is crucial for comprehending its chemical properties, magnetic behavior, and role in various applications, from catalysts to alloys. This comprehensive guide will delve into the process of determining Cobalt's electron configuration, explaining the underlying principles and providing practical examples.
Understanding Electron Configuration
Before diving into cobalt's specific configuration, let's establish a foundational understanding of the concept. Electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates how an atom will interact with other atoms, forming chemical bonds and exhibiting characteristic properties. The fundamental principles governing electron configuration include:
-
Aufbau Principle: Electrons fill orbitals starting with the lowest energy levels. This follows a predictable pattern based on increasing principal quantum number (n) and then increasing azimuthal quantum number (l).
-
Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins. This is represented by arrows pointing up and down in orbital diagrams.
-
Hund's Rule: When filling orbitals within a subshell (e.g., p, d, or f), electrons will individually occupy each orbital within that subshell before pairing up in any single orbital. This minimizes electron-electron repulsion.
Determining Cobalt's Electron Configuration
Cobalt (Co) has an atomic number of 27, meaning it has 27 protons and 27 electrons in a neutral atom. To determine its electron configuration, we'll systematically fill the orbitals according to the Aufbau principle, Pauli exclusion principle, and Hund's rule.
Step-by-Step Approach
-
Filling the lower energy levels: We begin by filling the lowest energy levels, which are the 1s, 2s, and 2p orbitals. These can accommodate a total of 10 electrons:
- 1s² (2 electrons)
- 2s² (2 electrons)
- 2p⁶ (6 electrons)
-
Moving to higher energy levels: Next, we move to the higher energy levels, filling the 3s and 3p orbitals:
- 3s² (2 electrons)
- 3p⁶ (6 electrons)
-
Transition metals and the 3d orbital: Now we encounter the 3d orbital. In general, the 4s orbital fills before the 3d, due to slight energy variations. However, it's important to understand this isn't always a strict rule; the energy difference is often small. The 3d orbital can hold up to 10 electrons. For Cobalt, we need to fill 7 electrons into the 3d subshell.
- 4s² (2 electrons)
- 3d⁷ (7 electrons)
Therefore, the complete electron configuration for a neutral cobalt atom is: 1s²2s²2p⁶3s²3p⁶4s²3d⁷
Orbital Diagram Representation
While the electron configuration provides a concise representation, an orbital diagram offers a more detailed visualization. Each orbital is represented by a box, and electrons are represented by arrows. For cobalt, the orbital diagram would appear as follows:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑↓ ↑↓ ↑↓
- 3s: ↑↓
- 3p: ↑↓ ↑↓ ↑↓
- 4s: ↑↓
- 3d: ↑ ↑ ↑ ↑ ↑ ↑↓
Notice that according to Hund's rule, the 3d orbitals are individually filled before any pairing occurs.
Exceptions and Subtleties in Electron Configuration
While the Aufbau principle provides a general guideline, certain exceptions exist, particularly among transition metals. The energy levels are not always perfectly spaced, and electron-electron repulsion can influence the final configuration. Cobalt, while largely following the Aufbau principle, demonstrates some of these subtleties. The relatively close energy levels of the 3d and 4s orbitals mean the precise electronic configuration can sometimes vary depending on the specific chemical environment or oxidation state of the cobalt atom.
Cobalt's Chemical Behavior and its Electron Configuration
The unique electron configuration of cobalt significantly influences its chemical properties and behavior. The presence of seven 3d electrons and two 4s electrons contribute to cobalt's ability to exhibit multiple oxidation states, typically +2 and +3. These oxidation states result from the variable loss of electrons from the 4s and 3d orbitals. This variable oxidation state makes cobalt a versatile element, enabling it to participate in a wide array of chemical reactions.
Furthermore, the partially filled 3d orbitals contribute to cobalt's magnetic properties. The unpaired electrons in these orbitals give rise to paramagnetism, meaning cobalt is attracted to external magnetic fields. This magnetic behavior is exploited in various technological applications, including magnets and magnetic storage devices.
Applications Leveraging Cobalt's Unique Properties
The unique electronic configuration of cobalt underlies its diverse applications. Here are some notable examples:
-
Alloys: Cobalt is a crucial component in various alloys, notably those used in high-strength, high-temperature applications. Its contribution to these properties stems directly from its electron configuration and the resulting metallic bonding interactions.
-
Catalysts: The ability of cobalt to readily adopt different oxidation states makes it an effective catalyst in several chemical processes. Its electron configuration allows for easy electron transfer during catalytic reactions.
-
Magnets: Cobalt is used in the creation of powerful permanent magnets, thanks to its paramagnetic properties linked to its partially filled d orbitals.
-
Medical Applications: Specific cobalt compounds find use in medical applications, often related to their catalytic or magnetic properties. These applications can involve imaging or treatment modalities.
Conclusion
Drawing the electron configuration for a neutral cobalt atom is a valuable exercise that demonstrates the fundamental principles governing electron arrangement within atoms. Understanding this configuration is vital for predicting and comprehending cobalt's chemical behavior, magnetic properties, and versatile applications. While the Aufbau principle provides a useful framework, remembering exceptions and subtle energy level variations are important for a deeper understanding. The electron configuration ultimately dictates cobalt's role in numerous scientific and technological advancements, showcasing the fundamental importance of atomic structure in determining macroscopic properties. This comprehensive guide has aimed to demystify this process, offering a step-by-step approach and insights into the significance of cobalt's unique electron configuration.
Latest Posts
Latest Posts
-
Carryover Cooking Will Continue To Cook An Item For About
Mar 21, 2025
-
During Its First Year Of Operations
Mar 21, 2025
-
Phrenology Highlighted The Presumed Functions Of
Mar 21, 2025
-
What Is The Password For The Companyinfo Zip File
Mar 21, 2025
-
The Government Engages In An Industrial Policy
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Draw The Electron Configuration For A Neutral Atom Of Cobalt . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
