Draw As Many Unique Lewis Structures As Possible For C4h8
Holbox
Mar 27, 2025 · 6 min read
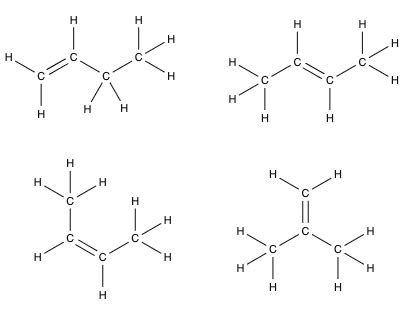
Table of Contents
- Draw As Many Unique Lewis Structures As Possible For C4h8
- Table of Contents
- Drawing Unique Lewis Structures for C₄H₈: A Comprehensive Guide
- Understanding Lewis Structures and Isomerism
- Structural Isomers of C₄H₈: Exploring the Possibilities
- 1. But-1-ene
- 2. But-2-ene
- 3. Methylpropene (or 2-Methylpropene)
- 4. Cyclobutane
- Stereoisomerism in But-2-ene: Cis and Trans (E and Z)
- Detailed Analysis of Lewis Structures: A Step-by-Step Approach
- Applications and Significance
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing Unique Lewis Structures for C₄H₈: A Comprehensive Guide
The hydrocarbon C₄H₈ presents a fascinating challenge for students learning about Lewis structures. Its molecular formula allows for several isomers, each with a unique arrangement of atoms and therefore a distinct Lewis structure. This article will systematically explore the various possibilities, delving into the principles of drawing Lewis structures and highlighting the key differences between each isomer. We'll go beyond simply providing the structures; we'll analyze their bonding, geometry, and implications for chemical properties.
Understanding Lewis Structures and Isomerism
Before diving into the structures of C₄H₈, let's briefly review the fundamentals. A Lewis structure, also known as an electron dot structure, is a visual representation of the valence electrons in a molecule. It shows how atoms are connected through covalent bonds and indicates the presence of lone pairs of electrons. The key to drawing accurate Lewis structures is understanding valence electrons and the octet rule (with some exceptions for elements like hydrogen).
Isomerism refers to the existence of molecules with the same molecular formula but different arrangements of atoms. These different arrangements, called isomers, lead to distinct chemical and physical properties. For C₄H₈, we encounter several types of isomerism, including structural isomerism (variations in the carbon skeleton) and stereoisomerism (variations in spatial arrangement).
Structural Isomers of C₄H₈: Exploring the Possibilities
C₄H₈ belongs to the alkene family of hydrocarbons, characterized by the presence of at least one carbon-carbon double bond. This double bond introduces possibilities for structural variations. Let's explore the different structural isomers systematically:
1. But-1-ene
-
Structure: The simplest isomer, but-1-ene, features the double bond at the end of the carbon chain. The Lewis structure shows a linear chain of four carbons, with a double bond between the first and second carbons. Each carbon atom forms four bonds (either single or double bonds with other carbons or single bonds with hydrogen atoms). All hydrogen atoms are bonded to carbon atoms.
-
Lewis Structure Representation:
H H H H | | | | H₂C=CH-CH₂-CH₃ -
Key Features: The presence of a terminal double bond influences its reactivity. It readily undergoes addition reactions across the double bond.
2. But-2-ene
-
Structure: But-2-ene places the double bond in the middle of the four-carbon chain.
-
Lewis Structure Representation:
H H H H | | | | H₃C-CH=CH-CH₃ -
Key Features: This isomer exhibits cis-trans (or E-Z) isomerism due to restricted rotation around the double bond. The cis isomer has the two methyl groups on the same side of the double bond, while the trans isomer has them on opposite sides. These isomers have different physical and chemical properties.
3. Methylpropene (or 2-Methylpropene)
-
Structure: This isomer has a branched carbon skeleton. The double bond is connected to a carbon atom that is bonded to three other atoms (one carbon and two hydrogens).
-
Lewis Structure Representation:
CH₃ | H₂C=C-CH₃ | H -
Key Features: The branching influences the molecule's steric hindrance and reactivity.
4. Cyclobutane
-
Structure: This is a cyclic isomer where the four carbon atoms form a closed ring. Each carbon atom is bonded to two other carbons and two hydrogen atoms.
-
Lewis Structure Representation:
CH₂ / \ CH₂ CH₂ \ / CH₂ -
Key Features: The cyclic structure introduces ring strain, affecting its stability and reactivity.
Stereoisomerism in But-2-ene: Cis and Trans (E and Z)
But-2-ene showcases stereoisomerism, specifically cis-trans isomerism (now more formally called E-Z isomerism based on Cahn-Ingold-Prelog priority rules). This arises from the restricted rotation around the carbon-carbon double bond.
-
Cis-But-2-ene (or Z-But-2-ene): The methyl groups are on the same side of the double bond.
-
Trans-But-2-ene (or E-But-2-ene): The methyl groups are on opposite sides of the double bond.
These two isomers are not easily interconverted under normal conditions, demonstrating that even though the connectivity remains the same, the three-dimensional arrangement leads to different properties. For example, trans-but-2-ene generally has a higher melting point than cis-but-2-ene because of its more symmetrical structure.
Detailed Analysis of Lewis Structures: A Step-by-Step Approach
Let's illustrate the step-by-step process of drawing the Lewis structure for But-1-ene:
-
Count Valence Electrons: Carbon has 4 valence electrons, and hydrogen has 1. For C₄H₈, the total number of valence electrons is (4 x 4) + (8 x 1) = 24.
-
Determine the Central Atoms: Carbon atoms typically form the backbone of organic molecules.
-
Connect Atoms with Single Bonds: Start by connecting the carbons with single bonds, forming a chain.
-
Add Remaining Electrons as Lone Pairs: Distribute the remaining valence electrons around the atoms to satisfy the octet rule (except for hydrogen, which only needs 2 electrons).
-
Form Double Bonds Where Necessary: If any atoms lack an octet, form double bonds to share electron pairs and satisfy the octet rule. In but-1-ene, a double bond is formed between C1 and C2.
-
Verify the Octet Rule: Ensure that each atom (except hydrogen) has eight valence electrons around it.
Applications and Significance
Understanding the various Lewis structures of C₄H₈ is crucial for several reasons:
-
Predicting Reactivity: The location of the double bond, the presence of branching, and the spatial arrangement all influence a molecule's reactivity. Knowing the Lewis structure allows us to predict how a molecule will react with different reagents.
-
Spectroscopic Analysis: Different isomers have distinct spectral signatures (in NMR, IR, and mass spectrometry). Their Lewis structures provide a framework for interpreting these spectra and identifying the specific isomer present in a sample.
-
Polymer Chemistry: Alkenes like but-1-ene and but-2-ene are important monomers in the synthesis of polymers like polybutene. The structure of the monomer directly affects the properties of the resulting polymer.
Conclusion
Drawing Lewis structures for C₄H₈ reveals a rich tapestry of isomeric possibilities, each with distinct properties. This exercise demonstrates the fundamental principles of bonding, isomerism, and the importance of visualizing molecular structure to understand chemical behavior. By systematically exploring these structures, we gain a deeper appreciation for the diversity and complexity of organic chemistry. The ability to confidently draw and interpret Lewis structures is a critical skill for success in chemistry and related fields. Remember that practice is key – the more Lewis structures you draw, the more confident and proficient you'll become.
Latest Posts
Latest Posts
-
Income Smoothing Describes The Concept That
Mar 31, 2025
-
When Can A Notary Submit An Application For Reappointment
Mar 31, 2025
-
A Financial Advisor Schedules An Introductory Meeting
Mar 31, 2025
-
Match Each Principal Function Of Management With Its Definition
Mar 31, 2025
-
Draw The Major Organic Product Of The Following Reaction
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Draw As Many Unique Lewis Structures As Possible For C4h8 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
