Draw A Structural Formula For 3-bromo-4-chloro-1 1-dimethylcyclohexane
Holbox
Mar 27, 2025 · 5 min read
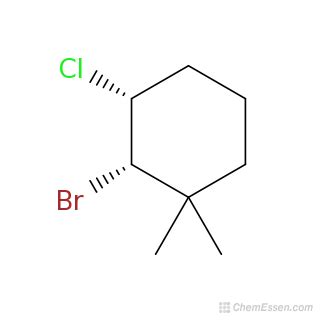
Table of Contents
- Draw A Structural Formula For 3-bromo-4-chloro-1 1-dimethylcyclohexane
- Table of Contents
- Drawing the Structural Formula for 3-Bromo-4-chloro-1,1-dimethylcyclohexane: A Step-by-Step Guide
- Understanding the IUPAC Nomenclature
- Step-by-Step Drawing Process
- Isomers and Stereoisomers
- Practical Applications and Further Exploration
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Drawing the Structural Formula for 3-Bromo-4-chloro-1,1-dimethylcyclohexane: A Step-by-Step Guide
Organic chemistry can feel daunting, especially when faced with complex molecule names like 3-bromo-4-chloro-1,1-dimethylcyclohexane. However, understanding the systematic naming conventions allows us to systematically deconstruct the name and accurately represent the molecule's structure. This guide provides a step-by-step approach to drawing the structural formula, explaining the logic behind each step and offering helpful tips for tackling similar challenges.
Understanding the IUPAC Nomenclature
The name "3-bromo-4-chloro-1,1-dimethylcyclohexane" follows the International Union of Pure and Applied Chemistry (IUPAC) nomenclature, a standardized system for naming organic compounds. Let's break down each part:
-
Cyclohexane: This is the parent hydrocarbon, a six-membered carbon ring (cyclo-) with single bonds (-ane). This forms the base of our structure.
-
1,1-dimethyl: This indicates two methyl groups (CH₃) attached to the same carbon atom (carbon 1). The "1,1" specifies their location on the cyclohexane ring.
-
3-bromo: A bromine atom (Br) is attached to carbon 3.
-
4-chloro: A chlorine atom (Cl) is attached to carbon 4.
The numbers (1, 3, and 4) indicate the positions of the substituents on the cyclohexane ring. We number the carbons in the ring to ensure the lowest possible numbers for the substituents.
Step-by-Step Drawing Process
Now, let's construct the structural formula step-by-step:
Step 1: Draw the Cyclohexane Ring
Begin by drawing a hexagon to represent the cyclohexane ring. Each corner represents a carbon atom, and each line represents a single bond. For clarity, it's helpful to explicitly show the carbon atoms at first, although later it's often omitted for brevity.
1
/ \
2---3
| |
6---4
\ /
5
Step 2: Add the Dimethyl Substituents
The name specifies "1,1-dimethyl," meaning two methyl groups are attached to the same carbon. Let's choose carbon 1 (you could start with any carbon; the numbering is arbitrary). Add two methyl groups (CH₃) to carbon 1.
1
/|\
2---3
| |
6---4
\ /
5
CH3 CH3
Step 3: Add the Bromo and Chloro Substituents
Next, add the bromine atom (Br) to carbon 3 and the chlorine atom (Cl) to carbon 4, as indicated by the name.
1
/|\
2---3 Br
| |
6---4 Cl
\ /
5
CH3 CH3
Step 4: Add Implicit Hydrogens (Optional)
While not always necessary, adding the implicit hydrogen atoms can further clarify the molecule's structure. Remember that each carbon atom in an organic molecule typically forms four bonds. Cyclohexane carbons have two bonds to neighboring carbons and two bonds to hydrogen atoms. Let's add those remaining hydrogens. Notice that carbon 1 already has its four bonds (two to methyl and two to cyclohexane carbons), so it doesn’t need hydrogens.
1
/|\
2---3-Br
| | |H
6---4-Cl |H
\ / |H
5 |H
CH3 CH3 H
Step 5: Condensed Structural Formula
For a more compact representation, we can use a condensed structural formula. In this representation, we omit some of the bonds but keep the connectivity clear.
CH3
|
CH3-C-CH2-CH(Br)-CH(Cl)-CH2
|
CH2
Step 6: Skeletal Formula (Line-Angle Formula)
The most concise way to represent this molecule is using a skeletal formula. In this representation, carbon atoms are implied at the corners and ends of lines. Hydrogen atoms bonded to carbon are not shown explicitly. The non-hydrogen atoms are drawn explicitly.
CH3
|
CH3-C-CH2-CH(Br)-CH(Cl)-CH2
|
CH2
Step 7: Chair Conformation (Stereochemistry)
Cyclohexane exists primarily in a chair conformation. The chair conformation minimizes steric hindrance. Representing the molecule in its chair conformation gives a more accurate representation of its 3D structure. The axial and equatorial positions of the substituents would need to be considered in this representation. Determining whether a group is axial or equatorial would require more advanced knowledge of conformational analysis.
(Note: A detailed drawing of the chair conformation would require a more advanced chemistry drawing tool and is beyond the scope of this textual explanation.)
Isomers and Stereoisomers
It's crucial to note that the name "3-bromo-4-chloro-1,1-dimethylcyclohexane" doesn't specify the stereochemistry. Therefore, several stereoisomers are possible. The molecule possesses chiral centers due to the presence of substituents on the cyclohexane ring, leading to different spatial arrangements of these substituents.
Practical Applications and Further Exploration
Understanding the structural formula of 3-bromo-4-chloro-1,1-dimethylcyclohexane is fundamental in several areas of chemistry:
-
Drug Discovery: Many drugs contain cyclohexane rings and halogen substituents. Understanding the structure-activity relationship is essential for designing and optimizing drug molecules.
-
Materials Science: Halogenated cyclohexanes can find use in polymer chemistry and material science applications.
-
Chemical Synthesis: The ability to draw and understand the structure is critical for planning and executing organic syntheses.
-
Spectroscopy: Knowing the structure aids in the interpretation of spectroscopic data, like NMR and IR spectroscopy, which are used to identify and characterize organic molecules.
Conclusion
Drawing the structural formula for 3-bromo-4-chloro-1,1-dimethylcyclohexane, while initially seeming complex, becomes straightforward with a systematic approach. By breaking down the IUPAC name, understanding the fundamental principles of organic chemistry, and following the steps outlined above, you can confidently represent this and similar molecules. This process not only helps in visualizing the molecule but is also crucial for understanding its properties, reactivity, and potential applications. Remember, practice is key to mastering organic chemistry nomenclature and structure drawing. Use various examples to solidify your understanding. Consider exploring online resources and interactive molecular visualization tools to further enhance your learning experience. The mastery of these skills is critical to progress in organic chemistry and related fields.
Latest Posts
Latest Posts
-
The Norton Field Guide To Writing 6th Edition
Mar 31, 2025
-
Label The Components Of The Baroreceptor Reflex
Mar 31, 2025
-
A Salad Bar Attendant Had To Replace An Empty Container
Mar 31, 2025
-
A Grant Application Has The Following Requirements
Mar 31, 2025
-
For Each Set Of Atoms Identify The Isotopes
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Draw A Structural Formula For 3-bromo-4-chloro-1 1-dimethylcyclohexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
