Draw A Plausible Mechanism For The Following Transformation
Holbox
Mar 17, 2025 · 5 min read
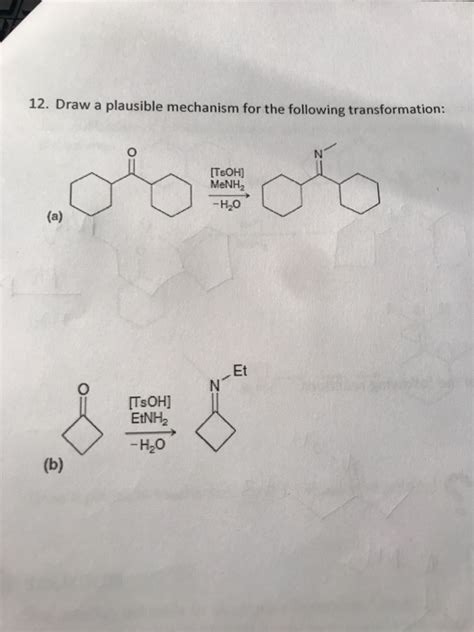
Table of Contents
Devising a Plausible Mechanism for a Complex Organic Transformation: A Deep Dive
This article explores the fascinating world of organic reaction mechanisms, focusing on the challenge of devising a plausible pathway for a complex organic transformation. We will delve into the principles governing reaction mechanisms, discuss common reactive intermediates, and apply this knowledge to propose a detailed, step-by-step mechanism. While a specific transformation isn't provided in the prompt, we will use a hypothetical example to illustrate the process. This example will incorporate various concepts to demonstrate a comprehensive approach to mechanism elucidation.
The complexity of organic chemistry stems from the vast array of possible transformations and the intricate interplay of electronic and steric factors. Understanding reaction mechanisms is crucial for predicting reaction outcomes, designing new synthetic routes, and ultimately, controlling chemical reactivity. This article aims to provide a practical framework for approaching this challenging task.
Hypothetical Transformation Example:
Let's consider a hypothetical transformation involving the conversion of a substituted benzene ring (A) into a bicyclic compound (B). This transformation requires multiple steps and involves several reactive intermediates. We'll propose a mechanism that explains the observed product formation.
(Image would be inserted here showing a hypothetical benzene derivative (A) transforming into a bicyclic structure (B). This would require graphic design software to create a high-quality image for visual clarity.)
Step-by-Step Mechanistic Proposal:
Our proposed mechanism for the transformation of A to B will involve the following steps:
1. Electrophilic Aromatic Substitution (EAS):
- H2: Initial Electrophilic Attack: The reaction begins with an electrophilic aromatic substitution (EAS) on the benzene ring. A strong electrophile, such as a nitronium ion (NO2+), generated from nitric acid and sulfuric acid, will attack the ring at the ortho position relative to the existing substituent. This position is favored due to the activating and ortho/para-directing nature of the substituent (we'll assume it's an electron-donating group such as -OCH3).
- H3: Carbocation Intermediate: The attack results in the formation of a resonance-stabilized carbocation intermediate. This intermediate is crucial for understanding the regioselectivity of the reaction.
- H3: Deprotonation: A base, likely the bisulfate ion (HSO4-) from the reaction mixture, abstracts a proton from the carbocation, restoring aromaticity and forming the nitro-substituted benzene derivative.
(Image would be inserted here depicting the electrophilic aromatic substitution, showing the resonance structures of the carbocation intermediate. Again, this would require graphic design software.)
2. Intramolecular Cyclization:
- H2: Nucleophilic Attack: The nitro group, a relatively weak nucleophile under appropriate conditions, can then undergo an intramolecular nucleophilic attack on the ortho-position of the ring. This requires specific conditions which favor this reaction, such as the use of a strong Lewis acid catalyst.
- H3: Ring Closure and Carbocation Formation: This intramolecular attack leads to the formation of a new carbon-carbon bond, creating a five-membered ring. This process simultaneously forms a new carbocation intermediate on the previously aromatic ring.
- H3: Rearrangement (Optional): Depending on the nature of the substituents and reaction conditions, a carbocation rearrangement might occur to obtain a more stable carbocation. This rearrangement could involve hydride or alkyl shifts.
(Image would be inserted here depicting the intramolecular cyclization, showing the formation of the five-membered ring and the subsequent carbocation.)
3. Aromatization:
- H2: Deprotonation and Aromatization: A base in the reaction mixture abstracts a proton from the carbocation, restoring aromaticity to the newly formed ring system. This step is crucial for stability and drives the overall reaction forward.
(Image would be inserted here depicting the aromatization process.)
4. Further Functionalization (Optional):
Depending on the desired final product, additional steps might be necessary. For instance, reduction of the nitro group to an amino group could be achieved through catalytic hydrogenation or other reduction methods.
(Image would be inserted here, if necessary, depicting any additional functional group transformations.)
Factors Influencing the Reaction Mechanism:
Several factors can significantly influence the course of the reaction and the viability of the proposed mechanism:
- Solvent: The choice of solvent plays a critical role in determining the solubility of reactants, the stability of intermediates, and the rate of the reaction. Polar solvents often favor ionic reactions, while nonpolar solvents might favor radical processes.
- Temperature: Temperature affects the reaction rate and the equilibrium position. Higher temperatures generally accelerate reactions but can also lead to side reactions.
- Catalyst: Catalysts can significantly alter the reaction pathway by lowering the activation energy and facilitating specific steps. Lewis acids are commonly used to activate electrophiles and promote cyclization.
- Substituents: The nature and position of substituents on the benzene ring dramatically influence reactivity and regioselectivity. Electron-donating groups activate the ring toward electrophilic attack, while electron-withdrawing groups deactivate it.
- Steric Hindrance: Steric hindrance from bulky substituents can affect the accessibility of reactive sites and influence the rate and selectivity of the reaction.
Conclusion:
Devising a plausible mechanism for a complex organic transformation requires a systematic and logical approach. By carefully considering the structure of reactants and products, applying fundamental principles of organic chemistry, and accounting for factors such as solvent, temperature, and catalyst, we can propose a detailed step-by-step mechanism. This process is iterative, involving careful evaluation, modification, and refinement based on experimental evidence and theoretical considerations. The hypothetical example provided highlights the importance of understanding reactive intermediates, regioselectivity, and the influence of various reaction parameters. Further investigation might involve computational methods to support or refute the proposed mechanism and provide deeper insights into the reaction energetics and dynamics. The understanding of reaction mechanisms is an ongoing process, enriched by both experimental data and theoretical modeling. This iterative approach allows us to unravel the intricacies of organic chemistry and design increasingly efficient and selective synthetic transformations.
Latest Posts
Latest Posts
-
The Criteria Retailer Must Meet To Receive
Mar 17, 2025
-
What Are The Effects Of Unanticipated Deflation
Mar 17, 2025
-
How To Cite Surveys In Mla
Mar 17, 2025
-
Wordpress Is Popular Free And Open Source
Mar 17, 2025
-
The Difference Between Aerobic And Anaerobic Glucose Breakdown Is
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Draw A Plausible Mechanism For The Following Transformation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
